A research team has developed a new synthesis method that can significantly reduce the sintering temperature required for the densification process of electrolytes in next-generation high-efficiency protonic ceramic cells. Their work is published in the journal Advanced Energy Materials.
Existing solid oxide cells (SOC) can produce electricity in fuel cell operation and hydrogen in electrolysis operation. Notably, they operate at high temperatures above 600°C, offering higher power conversion efficiency compared to other fuel cells. However, the downside is the high production cost due to the need for materials that can withstand high temperatures, as well as performance degradation over time due to thermal deterioration.
Recently, protonic ceramic cells (PCCs), which utilize proton (hydrogen ion) transport instead of oxygen ions, have emerged as next-generation energy conversion devices such as fuel cells and electrolyzers. Unlike conventional oxygen ion-conducting electrolytes, PCCs transport the smaller hydrogen ions, enabling higher ionic conductivity.
However, to produce the electrolyte for PCCs, sintering at temperatures above 1,500°C is required. During this process, component evaporation or precipitation occurs, degrading the electrolyte’s ion-conducting properties, which has been a major obstacle to the commercialization of PCCs.
The dual-phase proton ceramic electrolyte produced by the low-temperature synthesis process exhibits enhanced sintering characteristics, enabling a reduction in the sintering temperature of conventional processes. As a result, the intrinsic properties of the electrolyte can be realized in the device, improving cell performance. © Korea Institute of Science and Technology
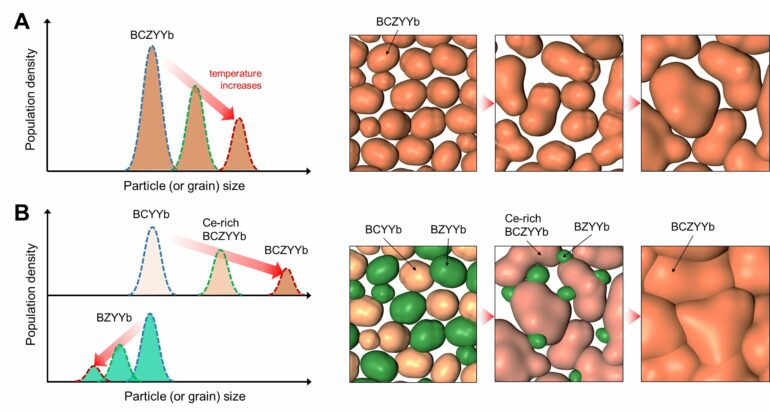
To lower the sintering temperature, the research team developed a new process for synthesizing electrolyte materials. Typically, the electrolyte for proton ceramic cells is produced by sintering a powder composed of a single compound. However, when additives are used to lower the sintering temperature, residual additives can remain in the electrolyte, reducing the cell’s power density.
The research team discovered that, by synthesizing a powder containing two different compounds through low-temperature synthesis, a single compound with excellent sintering properties forms during the sintering process accompanying the reaction to single phase. This allows the sintering temperature to drop to 1,400°C without the need for additives.
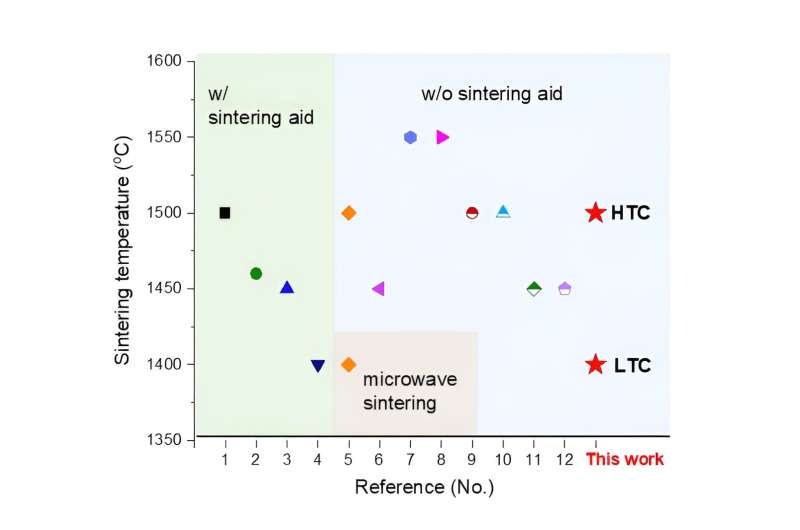
The new process, which does not use sintering aids or special sintering methods, achieved the lowest sintering temperature for electrolyte membranes. © Korea Institute of Science and Technology
The proton ceramic electrolyte synthesized through this new process forms a dense membrane even at lower temperatures, enhancing the electrochemical properties of the cell. When applied to actual proton ceramic cells, this electrolyte demonstrated superior proton conductance, achieving a power density of 950mW/cm2 at 600°C—approximately double that of existing cells.
This is expected to reduce process time and simultaneously improve thermal stability and the performance of ceramic electrolytes. The research team plans to apply this new process, which utilizes the accelerated sintering between the two compounds, to the production of large-area cells for the commercialization of proton ceramic cells.
The researchers included Dr. Ho-Il Ji from the Hydrogen Energy Materials Research Center at the Korea Institute of Science and Technology (KIST), along with Professor Sihyuk Choi’s team from Kumoh National Institute of Technology.
Dr. Ji of KIST stated, “This research has resolved the chronic sintering issues in the production of proton ceramic cells. If large-area technology is successfully developed, it will enable efficient energy management through green hydrogen production via electrolysis and pink hydrogen production by utilizing waste heat from nuclear power plants.”
More information:
Junseok Kim et al, Dual‐Phase Reaction Sintering for Overcoming the Inherent Sintering Ability of Refractory Electrolytes in Protonic Ceramic Cells, Advanced Energy Materials (2024). DOI: 10.1002/aenm.202400787
Provided by
National Research Council of Science and Technology
Citation:
Scientists develop a new electrolyte synthesis method for next-generation fuel cells (2024, October 10)