Korea Advanced Institute of Science and Technology (KAIST) researchers have developed a new hydrogen production system that will overcome the limitations of current green hydrogen production. It is expected that stable hydrogen production will be possible by utilizing a water-splitting system using a water-soluble electrolyte and blocking the risk of fire.
Professor Jeung Ku Kang’s research team in the Department of Materials Science and Engineering developed a self-powered hydrogen production system based on a high-performance zinc-air battery. The findings are published in the journal Advanced Science.
Hydrogen (H2) is a raw material for the synthesis of high value-added materials and is attracting attention as a clean fuel with an energy density (142 MJ/kg) that is three times higher than that of existing fossil fuels (gasoline, diesel, etc.). However, most current hydrogen production methods have the problem of emitting carbon dioxide (CO2).
In addition, green hydrogen production can be done by splitting water using renewable energy sources such as solar cells and wind power as the power source, but renewable energy-based power sources show low water splitting efficiency due to irregular power generation due to temperature, weather, etc.
To overcome this, air cells that can emit sufficient voltage (1.23 V or higher) for hydrogen production through water splitting are attracting attention as a power source, but precious metal catalysts must be used to achieve sufficient capacity, and there is a limitation that the performance of the catalyst material rapidly deteriorates during long-term charging and discharging.
Therefore, it is essential to develop a catalyst that is effective for water splitting reactions (oxygen generation, hydrogen generation) and a stable material for repeated charge and discharge reactions (oxygen reduction, oxygen generation) of zinc-air battery electrodes.
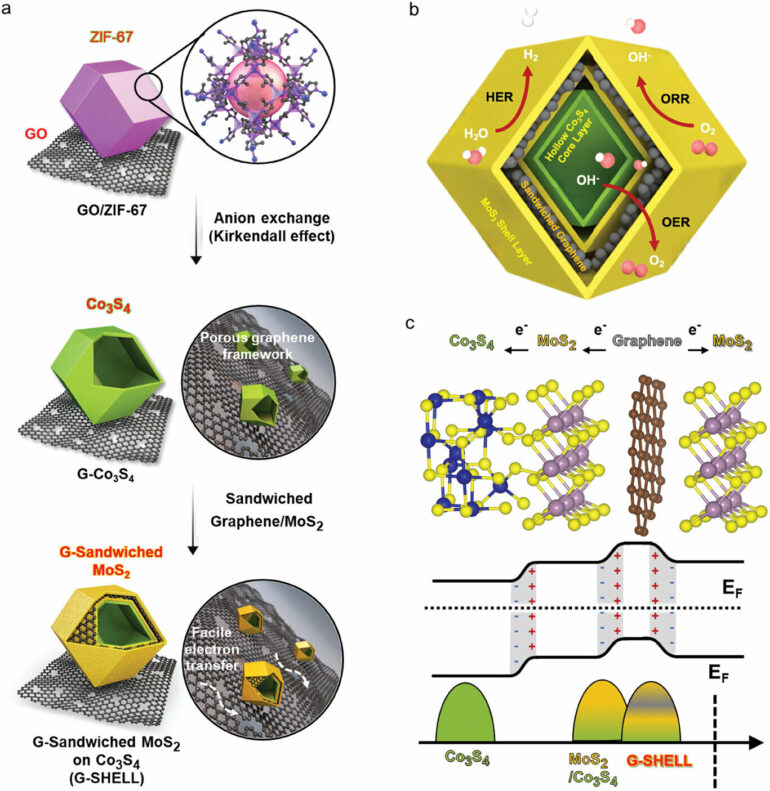
Accordingly, Professor Kang’s research team proposed a method for synthesizing a non-precious metal catalyst material (G-SHELL) that is effective for all three different catalytic reactions (oxygen generation-hydrogen generation-oxygen reduction) by utilizing a nano-sized metal-organic framework grown on graphene oxide.
The research team confirmed that the developed catalyst material was composed of the air electrode material of the air battery, and that it had an energy density (797 Wh/kg) that was about 5 times higher than that of existing batteries, high output characteristics (275.8 mW/cm²), and that it could operate stably for a long time even under repeated charging and discharging conditions.
In addition, the zinc-air battery, which is operated by a water-soluble electrolyte and is safe from the risk of fire, is expected to be applied as an eco-friendly method for hydrogen production by linking it with a water electrolysis system as a next-generation energy storage device.
Professor Kang said, “The zinc-air battery-based self-generation hydrogen production system, which was implemented by developing a catalyst material with high activity and lifespan in three different electrochemical catalytic reactions at low temperatures and in a simple manner, will be a new breakthrough that can overcome the limitations of current green hydrogen production.”
More information:
Dong Won Kim et al, Trifunctional Graphene‐Sandwiched Heterojunction‐Embedded Layered Lattice Electrocatalyst for High Performance in Zn‐Air Battery‐Driven Water Splitting, Advanced Science (2024). DOI: 10.1002/advs.202408869
Provided by
The Korea Advanced Institute of Science and Technology (KAIST)
Citation:
Self-powered hydrogen production system uses zinc-air battery to minimize fire risk (2024, October 23)