Current society is transitioning en masse from fossil fuels to renewable resources and electric batteries. Despite the urgency to switch to greener methods, core challenges related to efficiency and sustainability pose a hurdle to overcome. For instance, the mass market adoption of lithium-ion (Li-ion) batteries for use in electric vehicles is being hindered by their slow charging speeds. “Extreme” fast charging (wherein 80% of the battery is charged within 10 min), high energy density, and cycle life are the “holy grail” of features that the automobile industry seeks out in batteries.
In order to enable the fast-charging ability in batteries, researchers have long attempted to enhance the mass transfer of electrolytes and charge transfer in electrodes, with extensive research carried out on the former compared to the latter. Now, a study by a team of researchers, led by Professor Noriyoshi Matsumi from Japan Advanced Institute of Science and Technology (JAIST), showcases a new approach to facilitate fast charging using a binder material which promotes Li-ion intercalation of active material. The binder material leads to improved diffusion of desolvated Li ions across the solid electrolyte interface (SEI) and within the anode material and yields high conductivity, low impedance, and good stability.
The team comprised Former Senior Lecturer Rajashekar Badam, Postdoctoral Research Fellow Anusha Pradhan, Former Graduate Student Ryoya Miyairi, and Doctoral Course Student Noriyuki Takamori from JAIST. Their findings have been published in the journal ACS Materials Letters.
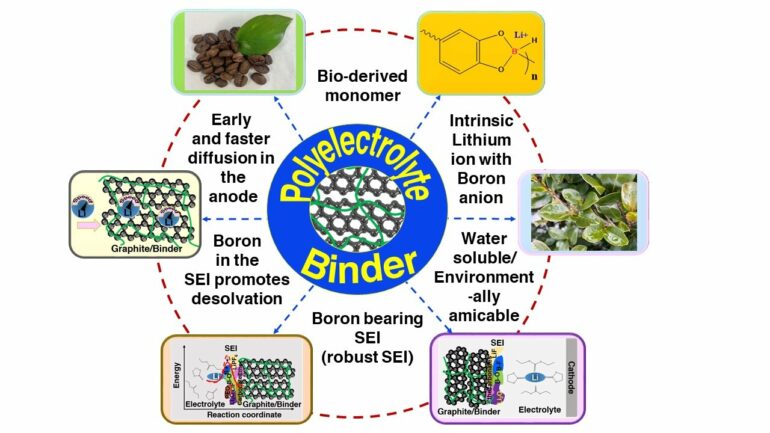
“Our current strategy of using a bio-derived lithium borate polymer as aqueous polyelectrolyte binder to enhance charge transfer within electrodes such as graphite anodes exhibits fast charging capability,” state corresponding authors Profs. Matsumi and Badam of JAIST.
While most research on batteries is focused on the design of active materials and improved mass transfer of electrolytes, the current study provides a different approach via the design of specific binder material which promotes lithium-ion intercalation of the active material. “The binder material includes highly dissociable lithium borate, which improves lithium-ion diffusion in the anode matrices. Further, this binder can form an organoboron SEI, which shows very low interfacial resistance when compared with ordinary battery cells,” explains Prof. Matsumi.
The role of boron compounds (such as the tetracordinate boron in the binder and the boron-rich SEI) is to aid in the desolvation of Li+ ions by decreasing the activation energy of desolvation of Li+ from the solvent sheath at the SEI. Also, with high diffusion and low impedance, the overpotential related to charge transfer at the interface is reduced. “This is one of the important determining factors for extreme fast charging,” explains Dr. Anusha Pradhan of JAIST, who is the first author of the paper.
Generally, when charging surpasses rate of intercalation, Li plating occurs on graphite electrodes. It is an undesired process leading to reduced battery life and limiting fast charge capability. In this study, the improved diffusion of ions across the SEI and within the electrodes limits the concentration polarization of Li+ ions—leading to the absence of plating on graphite.
In their study, not only do the researchers present a novel strategy for extremely high-rate chargeable batteries and reduced interfacial resistance, but they also used a biopolymer derived from caffeic acid. A plant-based organic compound, caffeic acid is a sustainable and environmentally safe source of material. Thus, while the market for these batteries grows tremendously, the use of bio-based resources in these batteries will also reduce carbon dioxide emissions.
Highlighting the key abilities of the structure used in this study, Prof. Matsumi adds, “In future studies, our binder can also be combined with high-rate chargeable active materials to enable further synergistic effect in enhancing performance.”
With increasing research into battery performance, one can soon look forward to greener options in the way we use energy, especially in the transportation sector. “Through the high-rate chargeable battery technology, people will enjoy electric vehicles and convenient mobile devices. As the use of renewable resources will maintain availability of products for [a] long [time], irrespective of availability of fossil resources and … social situations,” concludes Prof. Matsumi.
More information:
Anusha Pradhan et al, Extreme Fast Charging Capability in Graphite Anode via a Lithium Borate Type Biobased Polymer as Aqueous Polyelectrolyte Binder, ACS Materials Letters (2023). DOI: 10.1021/acsmaterialslett.2c00999
Provided by
Japan Advanced Institute of Science and Technology
Citation:
Speeding up extreme fast-charging capability in lithium-ion batteries (2023, March 3)