Chemical and material engineering professor, Adnan Khan, has spent the past 15 years focusing his research on developing sustainable technologies aimed at decarbonizing our energy systems. “This is the most important challenge we face today. We owe this to our future generations,” he says.
But it’s one thing to say we need to transition our energy systems, and another to figure out how to do it in a cost-effective and sustainable manner.
To help, Khan has created the Energy Transition Lab with the goal of developing and analyzing novel materials, technologies and credible transition pathways towards net-zero emissions for Canada.
Khan says the question of credibility is key for his 10-person lab, which employs systems-level analysis to examine whether new technologies or solutions will ultimately help or hinder the overall goal of energy transition.
“Before investing money, energy and time developing new materials and processes, we need to understand how a plant designed at a practical or industrial scale will work,” he says. “It is difficult to understand the challenges of the industrial process.”
This miscalculation of industrial-scale processes is the basis of a recent paper published by his team in the journal Nature Catalysis.The article shows that a well-intended emerging electrification technology that is designed to help decarbonize the chemical sector—which accounts for two percent of global emissions—will actually increase both energy use and cost.
“Decarbonizing the industry using electrification is a step in the right direction, but the technology researchers are currently pursuing can lead to significant challenges,” Khan says.
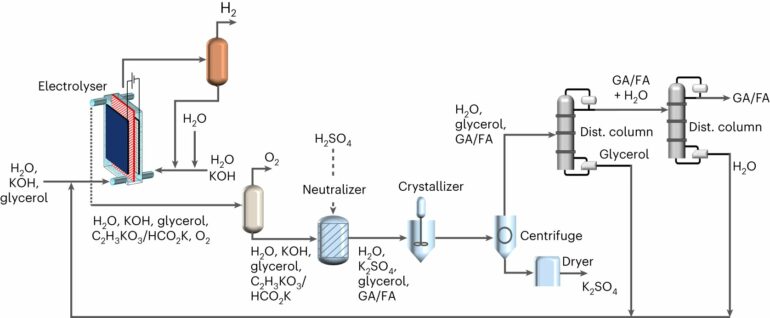
The technology is based on alkaline electrocatalytic systems that can be powered by renewable electricity for chemical production. These systems employ a basic solution with a high pH, or high alkalinity, to speed up chemical reactions that involve electricity.
The team shed light on the challenges of developing an industrial process based on this technology, particularly the downstream product separation. To do it, they simulated the industrial process and analyzed it using economic models they developed in-house.
For the study, Khan looked at the process to produce glycolic acid. Glycolic acid is a valuable chemical used in the cosmetics industry and the household and industrial cleaning industry. It is touted to play a key role in the future of bioplastics.
The results presented in the study suggest that an alkaline electrocatalytic route for glycolic acid production would require the use of expensive downstream product separation that would lead to an increase in both raw material and energy costs. All told, the price of producing glycolic acid produced this way would jump to US$2.20 to 2.50 per kilogram, an increase of up to 40% compared to the current market price, which is around US$1.50 per kilogram.
Khan concludes that the challenges related to acid-base reaction chemistry are often overlooked at the catalyst development stage, resulting in a significant waste of research resources.
“I know we have to act fast because human activity-related emissions have led to a significant impact on climate change. But we need to develop credible solutions to get to net-zero emissions.”
More information:
Shariful Kibria Nabil et al, Acid–base chemistry and the economic implication of electrocatalytic carboxylate production in alkaline electrolytes, Nature Catalysis (2024). DOI: 10.1038/s41929-024-01107-6
Provided by
University of Alberta
Citation:
Finding credible pathways to net-zero emissions: The challenge of scaling up an emerging electrification technology (2024, May 16)