The hypothalamus is involved during the coordination of neuroendocrine functions in vertebrates and their evolutionary origin can be described using integrated transcriptome or connectome brain maps of swimming tadpoles of Ciona intestinalis, also known as sea vase. These organisms serve as an approximation of their ancestral protovertebrate. The map included several cell types relative to different regions of the vertebrate hypothalamus, including the mammillary nucleus, arcuate nucleus and magnocellular neurons. These observations highlighted how the hypothalamus predates the evolution of the vertebrate brain. The neural crest and cranial placodes are key innovations that contributed to the evolution of the vertebrate head. However, less is known about the evolutionary origin of the crown and summit of the vertebrate brain. In a new study now on Science Advances, Laurence A. Lemaire and a research team in molecular biology and integrative genomics at the Princeton University, New Jersey, U.S., used an extensive single-cell transcriptome fate map of the Ciona tadpole to characterize the neural cell types comprising its brain also known as the sensory vehicle.
The hypothalamus
The sensory vesicle of the Ciona tadpole contains 215 neural cells including 143 neurons and is primarily responsible to relay sensory information including light, gravity and mechanical cues to the motor ganglion that controls the tadpole tail. The central nervous system (CNS) of the Ciona has facilitated lineage maps to allow the first comprehensive connectome of a chordate. In an attempt to include the synaptic connectome, Lemaire et al. studied the evolutionary origins of the vertebrate brain, specifically the hypothalamus. The hypothalamus has ancient origins and forms an ancient region of the vertebrate brain. The construct is found across all vertebrates including fish to humans, the hypothalamus controls homeostasis, metabolism and reproductive functions through intricate interconnecting neural circuits. In this work, Lemaire et al. propose the major function of the Ciona proto-hypothalamus to be to trigger the onset of metamorphosis of the tadpole species.
Brain map
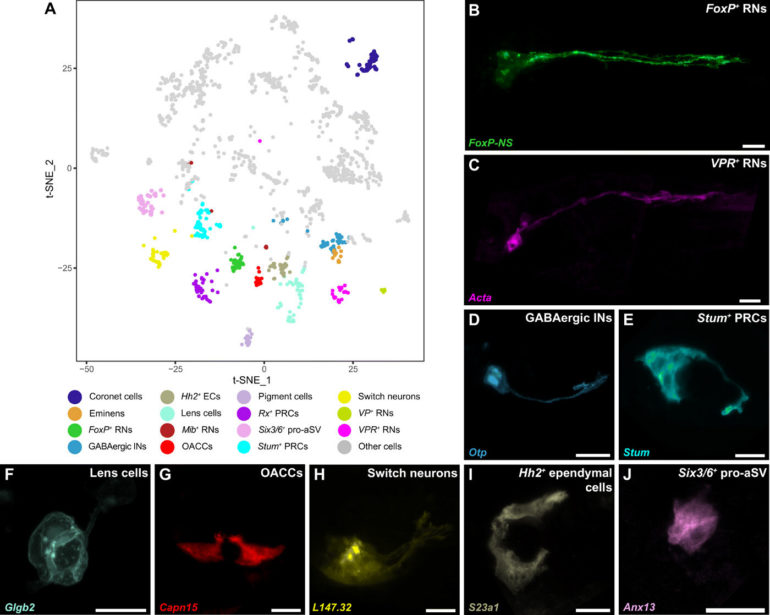
The scientists conducted single-cell transcriptome profiling of the Ciona intestinalis embryogenesis from gastrulation to swimming larvae, to identify 40 t-distributed stochastic neighbor embedding (t-SNE) cell clusters of the CNS (central nervous system) and peripheral nervous system. Lemaire et al. mapped each of the neural cell types comprising the simple brain of the tadpoles also known as their sensory vesicles. Based on the studies, they identified 15 different neural cell types including previously identified coronet cells, Eminens neurons and pigment cells. Using neural-specific reporter genes, the team identified a range of relay neurons (RNs) including those that expressed vasopressin and tachykinin (FoxP+), as well as others expressing the vasopressin receptor (VPR+). The FoxP+ relay neurons were cholinergic while the VPR+ relay neurons were GABAergic. The researchers identified an underappreciated population of putative mechanosensory neurons, which they renamed ‘switch neurons,” corresponding to ciliated brain interneurons in the connectome map of the CNS of tadpoles.
Neuronal circuit
Scientists had previously identified similarities of coronet cells with the vertebrate hypothalamus since they released dopamine to express diverse neuropeptides including neurotensin-like B and gonadotropin-releasing hormone (Gnrh). However, these cells were previously described to share morphological similarities with the coronet cells or a region of the hypothalamus present in non-tropical fish. The fish coronet cells also expressed melanopsin and detected a short wavelength light associated with seasonal lengthening of daylight to trigger reproduction by releasing a thyroid-stimulating hormone, followed by the secretion of Gnrh (gonadotropin-releasing hormone) as in other seasonal breeders. The work showed how coronet cells functioned as light-sensing sensory cells relative to their dopaminergic and neurosecretory activities. The coronet cells also interacted with adjacent neurons such as switch neurons and FoxP+ relay neurons. On the basis of their anatomical position, the switch neurons corresponded to ciliated brain interneurons. On the basis of cell-cell associations, the VPR+ relay neurons also received inputs from switch neurons.
Mechanosensory switch neurons
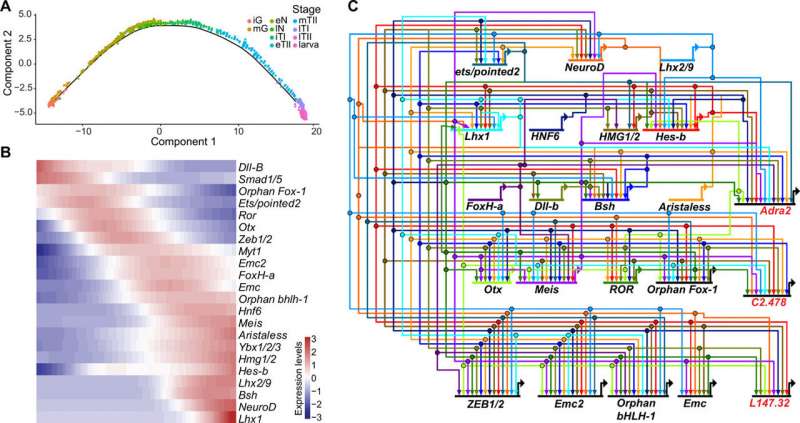
Previous studies had shown coronet cells to be a central sensory node for associated neurons and FoxP+ relay neurons. While detailed information currently exists on the networks that underly the specifications of coronet cells, not much is known about the development of switch neurons or FoxP+ relay neurons. Lemaire et al. therefore focused on switch neurons due to their roles as specialized mechanosensory cell types in vertebrates, including the cerebrospinal fluid containing neurons present along the central canal and the ventricular cavities of the brain including the hypothalamus. Additionally, not much is also known about the development or function of vertebrate cerebrospinal fluid contacting neurons (CSF-cNs). To understand their ontogeny, Lemaire et al. created a provisional gene regulatory network for switch neurons, using previously published methods. The team identified transcriptome trajectories and temporal cascades of genes encoding transcription factors in the cell lineages to form switch neurons. They represented the resulting interconnections as a provisional gene network. As proof of concept, they used single-cell RNA sequencing (scRNA-seq) assays to understand the cell types that are transformed into switch neurons after the mis-expression of a gene, to test the authenticity of the network.
Orthology maps of mouse hypothalamus and Ciona nervous system
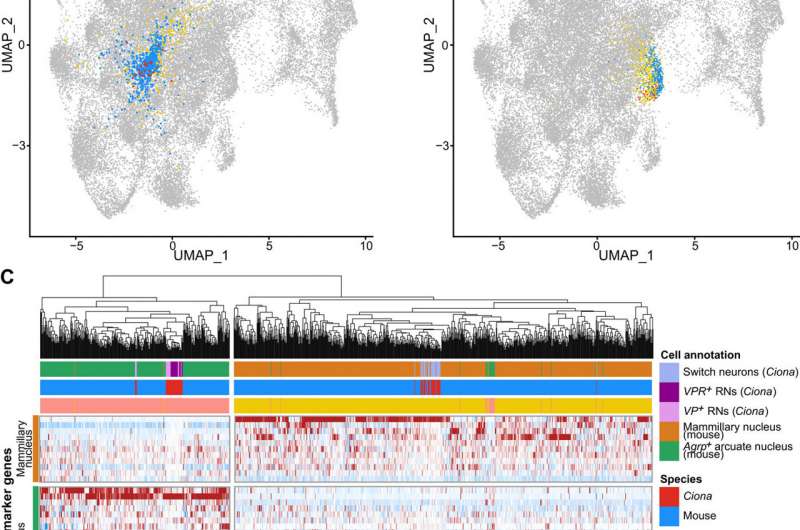
The team formed a putative sensory circuit featuring coronet cells as a central node, in association with switch mechanosensory neurons and the relay neurons. Existing studies alongside the current demonstration of coronet cells that express melanopsin and pinopsin provided considerable evidence for the homology with the vertebrate hypothalamus. To test if the relay neurons and the associated switch might share homology with the hypothalamus, Lemaire et al. performed whole-transcriptome for each of the 40 neural cell types comprising the Ciona nervous system and compared them with the transcriptome maps of the mouse hypothalamus. The studies identified two Ciona lineages that matched two different clusters of mouse hypothalamic cells. The combined comparative transcriptome analyses suggested the coronet-associated neural circuit to contain multiple cell types relative to different regions of the mouse hypothalamus.
Outlook
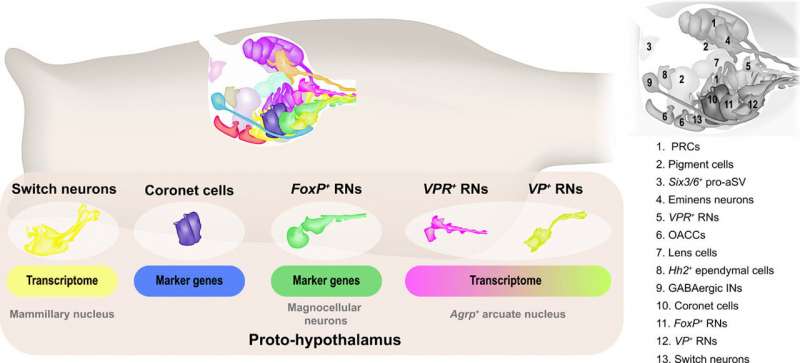
In this way, Laurence A. Lemaire and colleagues identified 15 different cell types in the sensory vesicle of Ciona larvae, while the connectome map identified 31 cell types. The team credited this disparity to reflect the different methods of classification. For instance, they noted how a single cell type based on intrinsic genetic properties could acquire distinctive behaviors through associations with different neurons. The scientists described five different types of relay neurons based on the transcriptome trajectories and profiles, while the connectome map identified 11 such neurons relative to synaptic inputs. The outcomes suggested the simple brain morphology of Ciona to contain a complex proto-hypothalamus with a role during the onset of metamorphosis in the tadpoles. Regardless of the intended function, this work indicates the evidence of multiple hypothalamic cell types in Ciona to suggest an unexpectedly sophisticated blueprint for the evolution of the complex vertebrate brain.
Scientists reveal origin of neuronal diversity in hypothalamus
More information:
Lemaire A. L. et al. The hypothalamus predates the origin of vertebrates, Science Advances, 10.1126/sciadv.abf7452
Abitua P. B. et al. Identification of a rudimentary neural crest in a non-vertebrate chordate. Nature, doi.org/10.1038/nature11589
Kindt K. S. et al. Caenorhabditis elegans TRPA-1 functions in mechanosensation. Nature Neuroscience, doi.org/10.1038/nn1886
2021 Science X Network
Citation:
The hypothalamus predates the origin of vertebrates (2021, May 28)
retrieved 30 May 2021
from https://phys.org/news/2021-05-hypothalamus-predates-vertebrates.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.