If you are a frequent flyer, you’ve probably been at the airport waiting to jet somewhere on a winter trip when the voice of an airline employee announces over the intercom that there will be a slight delay while the plane gets deiced. But how does this process actually work, and why is it needed?
As a mechanical engineer who studies frost growth and water droplets on surfaces, I have come to appreciate the importance of deicing planes. Indeed, deicing is an important safety step performed by the airlines on wintry days because of how snow and ice can affect the physics of flying.
Why deice?
In short, deicing is necessary because snow and ice on airplane wings can decrease lift by as much as 30%. Lift is the vertical upward force that keeps a plane in the sky. It is generated when air flows over the wings of a plane.
Ice and snow can alter how air flows over the wings, which can affect a pilot’s ability to maneuver and control the aircraft. It can also increase the stall speed, which is not good either. Stall speed is the minimum speed needed by an aircraft to generate enough lift to keep it aloft.
Additionally, ice on the wings can break off in flight, potentially damaging one or more of the flaps on the wings or an engine. Needless to say, deicing has become an indispensable part of flying, especially in the winter months.

Operators apply green anti-icing fluid to the wing of a plane. The green hue, which indicates a Type IV fluid, helps the operators see which parts they might have missed.
Orchidpoet/E+ via Getty Images
Deicing chemicals
Most people are familiar with the chemical deicers that are used on roads during the winter months. However, the salts in these products can be corrosive, so they’re not used on aircraft.
Aircraft deicers consist of a water-based solution of glycol – a colorless, odorless organic liquid – mixed with various additives. These additives might include a thickening agent; a substance that prevents corrosion; a surfactant, which decreases the surface tension; a flame retardant, and a dye.
Glycols are very good at lowering the freezing point of water, which makes it harder for water to freeze or stay frozen on surfaces. Propylene glycol and ethylene glycol are the two most common types used, typically making up 30% to 70% of the deicing solution.
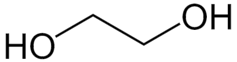
Glycols are made up of carbon, hydrogen and oxygen atoms. Pictured here is the chemical structure of ethlyene glycol.
Cacycle/Wikimedia Commons, CC BY-SA
For years, only ethylene glycol was used in deicers because of its low cost. However, because propylene glycol is less toxic to wildlife and humans, its adoption by commercial airlines has grown steadily since the 1980s.
How does the deicing process work?
Airlines use four standard fluid types when deicing aircraft. These fluids have different viscosities – viscosity is a measure of a fluid’s resistance to…