Even as a hollow ball of embryonic cells, developing fish and mammals are not entirely defenceless.
The very first tissue, formed on the surface of a vertebrate blastula, has been shown to possess an innate immune response.
Incredible new research has shown that long before the development of organs or specialized immune cells, this simple protective layer, known as the epithelium, can reach out with its arm-like protrusions and detect, ingest, and destroy defective cells – helping to increase the embryo’s chance of survival.
This ‘surprisingly’ efficient process, which was filmed in zebrafish and later confirmed in mice, is the earliest sign of an immune response in vertebrates.
Better understanding how it works could help researchers figure out why some embryos fail to form in those earliest states, and potentially lead to new approaches for treating infertility or early miscarriages.
“Here we propose a new evolutionarily conserved function for epithelia as efficient scavengers of dying cells in the earliest stages of vertebrate embryogenesis,” says cell biologist Verena Ruprecht from the Centre for Genomic Regulation.
“Our work may have important clinical applications by one day leading to improved screening methods and embryo quality assessment standards used in fertility clinics.”
In developing animals, it’s not uncommon for embryos to produce cellular errors during rapid cell division, and these can cause the whole embryo to fail if not taken care of. In fact, such mistakes are thought to be a leading reason for why embryos do not survive to reach implantation.
Scientists have long suspected there is an innate immune response at play, keeping fragile young embryos from threats such as sporadic cell death, inflammation, and infectious agents.
Recent research revealed such innate immune responses in both mouse and human embryonic stem cells. But up until now, no one had ever seen it in action.
This newest study is the first to explain how ‘garbage collectors’ like apoptotic cells are cleared out of the blastula without a specialized immune system. As you can see in the footage below, it looks a little like PAC-MAN.
So how does it work?
The blastula is a hollow ball, one cell thick, and the first stage of embryogenesis. The next stage includes further division into three germ layers, known as the gastrula.
In both these preliminary stages, researchers found evidence for the clearance of apoptotic cells, which initiate cell death.
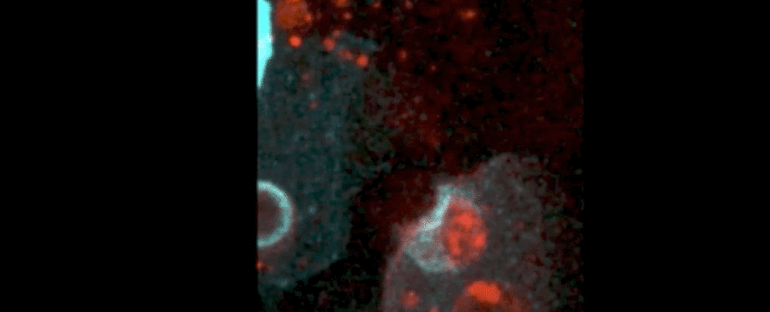
Using four dimensional in vivo imaging of mice and zebrafish embryos, the authors show two types of epithelial ‘arms’ that seem to gobble up and destroy these apoptotic cells.
The first protrusion is called a phagocytic cup, and it helps scoop up and swallow the apoptotic target, a process known as phagocytosis. This structure is not unlike what we see in adult organisms, where epithelial phagocytosis keep organs and tissues healthy from infection and inflammation.
The second protrusion is a previously undescribed structure that is fast and can mechanically push apoptotic targets around, herding them into manageable positions.
“The cells cooperate mechanically,” explains developmental biologist Esteban Hoijman, “like people distributing food around the dining table before tucking into their meal, we found that epithelial cells push defective cells towards other epithelial cells, speeding up the removal of dying cells.”
Three dimensional tracking of these defective cells show they actually accumulate inside the epithelium, which suggests this protective layer is singling out certain cells specifically and gulping them up.
Even in conditions with abundant apoptosis, or cell death, occurring, zebrafish embryos were able to survive, which suggests this immune response is a highly efficient one.
Within two hours, in fact, the authors found the embryonic epithelium could remove 68 apoptotic particles.
Even when programmed cell death was triggered in the blastula using only two photons of illumination, the embryo showed epithelial clearance, indicating an impressive level of sensitivity.
“Together, these observations establish epithelial clearance as an error-correction mechanism that is present at the blastula stages of embryonic development,” the authors conclude.
Zebrafish are model organisms for studying embryonic development, but to see whether this ‘epithelial scavenging’ also stood in mammals, the authors investigated what cell death looks like in mouse blastocysts.
Through time lapse imaging, the results reveal several apoptotic events, whereby cells are forced out of the blastocyst cavity and later ingested by the trophoblast. This is a tissue on the outside of the mammalian embryo that later forms a large part of the placenta. It also shows some level of innate immune response.
When mouse blastocysts were transplanted with apoptotic embryonic stem cells, the authors observed trophoblast cells eating up the targets.
Similar functions have also been documented in the human trophectoderm, which suggests the phagocytic epithelium has also been conserved in mammals and doesn’t just appear in fish.
Knowing how mammal embryos survive from blastocyst to implantation could not only allow scientists to develop better fertility treatments, it could also teach us something about the early immune system – a power we could possibly try to replicate in adult tissues.
“Here we show that during early vertebrate development, epithelial cells specialize to perform phagocytic immune functions in the complete absence of immune cells,” the authors write.
“At later developmental stages, professional phagocytes differentiate and can share their phagocytic tasks with mesenchymal or epithelial cells.”
Future research will determine if the same innate immune process is also observed in invertebrates.
The study was published in Nature.