There’s always more to explore and understand. It holds true if you zoom out to the far reaches of the Universe, or if you zoom in on tiny organisms. In science, the more questions you answer, the more you discover that needs to be asked.
And so, researchers have taken their high-powered microscopes to the protective ‘skin’ of bacteria, peering down into the depths of how this membrane is organized, revealing more detail than ever before.
Gram-negative bacteria like Escherichia coli have outer membranes to hold their innards in place, and protect them from the hustle and bustle of bacterial life. These membranes have a fabulous collection of tools, including outer-membrane proteins and toxins like lipopolysaccharides studding the surface.
But once you know this, more questions arise – how does all this fabulousness fit together? What’s the ratio of ‘studs’ to membrane across the whole bacterium? This is where the new research comes in.
“The outer membrane is a formidable barrier against antibiotics and is an important factor in making infectious bacteria resistant to medical treatment. However, it remains relatively unclear how this barrier is put together, which is why we chose to study it in such detail,” explained University College London biophysicist and senior author Bart Hoogenboom.
“By studying live bacteria from the molecular to cellular scale, we can see how membrane proteins form a network that spans the entire surface of the bacteria, leaving small gaps for patches that contain no protein.”
The researchers used an atomic force microscope for this task, which is a microscope that continuously pokes the surface of the membrane to determine what the shape looks like. It’s a little like reading braille, except with lasers instead of fingertips.
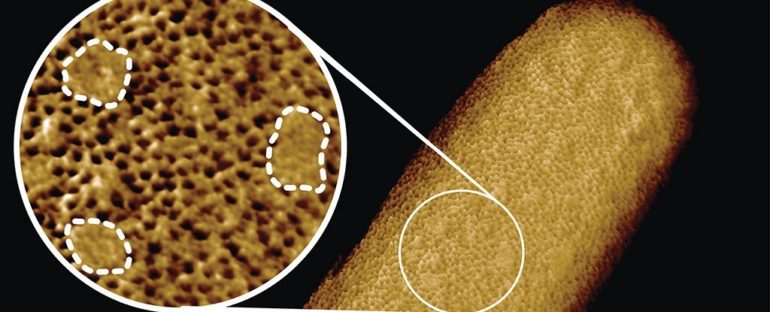
The team then produced a wonderful image of the bacterial membrane – which they call the sharpest images ever of living bacteria – showing the density of the outer-membrane proteins across the surface.
Atomic force microscopy scans showing the outer membrane. (Benn et al., PNAS, 2021)
As you can see in the image above, there are lots of tiny holes across the surface. These are beta barrel proteins, called porins, that form tunnels through the bacteria’s membrane to allow molecules to diffuse through. Meanwhile, the small sections of smooth surface are the lipopolysaccharides which can expand as the cell grows.
“We conclude that the outer membrane is a mosaic of phase-separated lipopolysaccharide-rich and outer-membrane protein-rich regions, the maintenance of which is essential to the integrity of the membrane and hence to the lifestyle of a gram-negative bacterium,” the team writes in the new paper.
Of course, scientists don’t stare at E. coli with huge microscopes just for the fun of it, as nice as that would be. There are good reasons for us to need this level of detailed understanding.
“The textbook picture of the bacterial outer membrane shows proteins distributed over the membrane in a disordered manner, well-mixed with other building blocks of the membrane. Our images demonstrate that that is not the case, but that lipid patches are segregated from protein-rich networks just like oil separating from water, in some cases forming chinks in the armor of the bacteria,” explains first author and UCL biochemist Georgina Benn.
“This new way of looking at the outer membrane means that we can now start exploring if and how such order matters for membrane function, integrity and resistance to antibiotics.”
The team will be investigating how we can use this new knowledge to outsmart antimicrobial resistance in gram-negative bacteria such as E. coli.
Like the stars or black holes in far flung galaxies, once you’ve found something, there are always more questions to answer.
The research has been published in PNAS.