American and Polish scientists, reporting Oct. 16 in the journal Science Advances, laid out a novel rationale for COVID-19 drug design—blocking a molecular “scissor” that the virus uses for virus production and to disable human proteins crucial to the immune response.
The researchers are from The University of Texas Health Science Center at San Antonio (UT Health San Antonio) and the Wroclaw University of Science and Technology. Information gleaned by the American team helped Polish chemists to develop two molecules that inhibit the cutter, an enzyme called SARS-CoV-2-PLpro.
SARS-CoV-2-PLpro promotes infection by sensing and processing both viral and human proteins, said senior author Shaun K. Olsen, Ph.D., associate professor of biochemistry and structural biology in the Joe R. and Teresa Lozano Long School of Medicine at UT Health San Antonio.
“This enzyme executes a double-whammy,” Dr. Olsen said. “It stimulates the release of proteins that are essential for the virus to replicate, and it also inhibits molecules called cytokines and chemokines that signal the immune system to attack the infection,” Dr. Olsen said.
SARS-CoV-2-PLpro cuts human proteins ubiquitin and ISG15, which help maintain protein integrity. “The enzyme acts like a molecular scissor,” Dr. Olsen said. “It cleaves ubiquitin and ISG15 away from other proteins, which reverses their normal effects.”
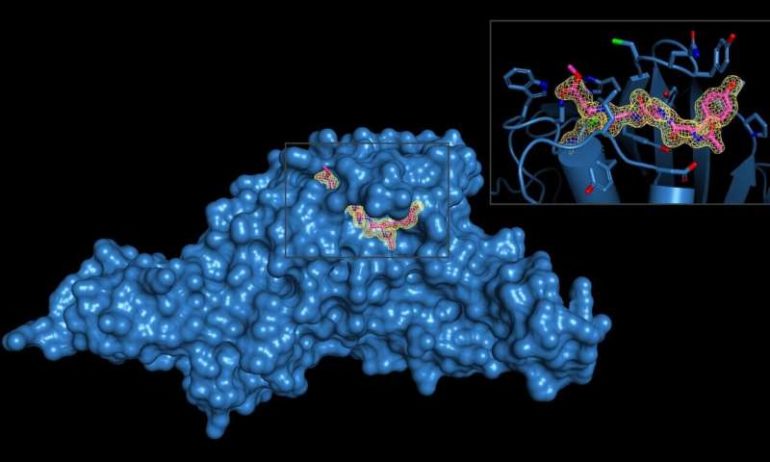
Dr. Olsen’s team, which recently moved to the Long School of Medicine at UT Health San Antonio from the Medical University of South Carolina, solved the three-dimensional structures of SARS-CoV-2-PLpro and the two inhibitor molecules, which are called VIR250 and VIR251. X-ray crystallography was performed at the Argonne National Laboratory near Chicago.
“Our collaborator, Dr. Marcin Drag, and his team developed the inhibitors, which are very efficient at blocking the activity of SARS-CoV-2-PLpro, yet do not recognize other similar enzymes in human cells,” Dr. Olsen said. “This is a critical point: The inhibitor is specific for this one viral enzyme and doesn’t cross-react with human enzymes with a similar function.”
Specificity will be a key determinant of therapeutic value down the road, he said.
The American team also compared SARS-CoV-2-PLpro against similar enzymes from coronaviruses of recent decades, SARS-CoV-1 and MERS. They learned that SARS-CoV-2-PLpro processes ubiquitin and ISG15 much differently than its SARS-1 counterpart.
“One of the key questions is whether that accounts for some of the differences we see in how those viruses affect humans, if at all,” Dr. Olsen said.
By understanding similarities and differences of these enzymes in various coronaviruses, it may be possible to develop inhibitors that are effective against multiple viruses, and these inhibitors potentially could be modified when other coronavirus variants emerge in the future, he said.
Follow the latest news on the coronavirus (COVID-19) outbreak
More information:
Activity profiling and structures of inhibitor-bound SARS-CoV-2-PLpro protease provides a framework for anti-COVID-19 drug design, Science Advances (2020). DOI: 10.1126/sciadv.abd4596
Provided by
University of Texas Health Science Center at San Antonio
Citation:
Viral ‘molecular scissor’ is next COVID-19 drug target (2020, October 16)
retrieved 16 October 2020
from https://medicalxpress.com/news/2020-10-viral-molecular-scissor-covid-drug.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.