It’s a paradox: Life needs water to survive, but a world full of water can’t generate the biomolecules that would have been essential for early life. Or so researchers thought.
Water is everywhere. Most of the human body is made of it, much of planet Earth is covered by it and humans can’t survive more than a couple of days without drinking it. Water molecules have unique characteristics that allow them to dissolve and transport compounds through your body, provide structure to your cells and regulate your temperature. In fact, the basic chemical reactions that enable life as we know it require water, photosynthesis being one example.
However, when the first biomolecules like proteins and DNA started coming together in the early stages of planet Earth, water was actually a barrier to life.
The reason why is surprisingly simple: The presence of water prevents chemical compounds from losing water. Take, for example, proteins, which are one of the main classes of biological molecules that make up your body. Proteins are, in essence, chains of amino acids linked together by chemical bonds. These bonds are formed through a condensation reaction that results in the loss of a molecule of water. Essentially, the amino acids need to get “dry” in order to form a protein.
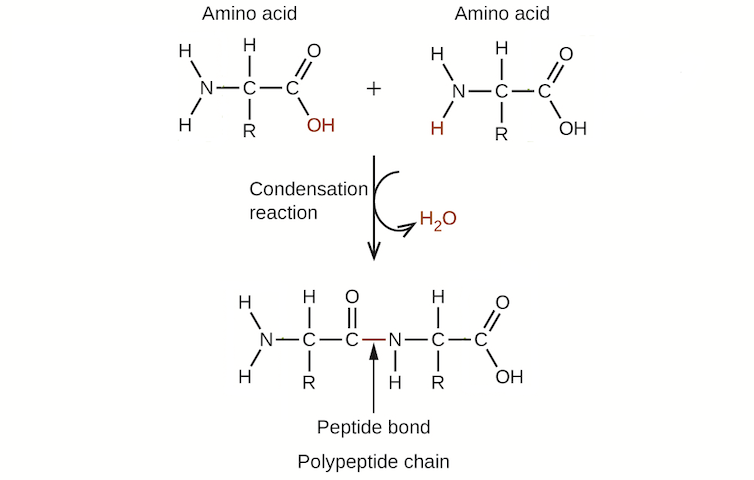
Condensation reactions join amino acids by losing a molecule of water.
OpenStax/Wikimedia Commons, CC BY
Considering that Earth before life was covered in water, this was a big problem for making the proteins essential to life. Like trying to get dry inside of a swimming pool, two amino acids would have had a hard time losing water to come together in the primordial soup of early Earth. And it wasn’t only proteins that faced this problem in the presence of water: Other biomolecules essential to life, including DNA and complex sugars, also rely on condensation reactions and losing water to form.
Over the years, researchers have proposed many solutions to this “water paradox.” Most of them rely on very specific scenarios on early Earth that could have allowed water removal. These include drying puddles, mineral surfaces, hot springs and hydrothermal vents, among others. These solutions, while plausible, require particular geological and chemical conditions that might not have been commonplace.
In our recent study, my colleagues and I found a simpler and more general solution to the water paradox. Quite ironically, it might be water itself – or to be more precise, very small water droplets – that allowed early biomolecules to form.
Why microdroplets?
Water droplets are everywhere, both in the modern world and especially during prebiotic (or pre-life) Earth. In a planet covered by crashing waves and raging tides, the small water droplets in sea spray and other aerosols would have plausibly provided a simple and abundant place for the first biomolecules to assemble.
Water microdroplets – typically very small droplets…