Curious Kids is a series for children of all ages. If you have a question you’d like an expert to answer, send it to [email protected].
“What is radium and why is it dangerous?” – Aurora, 10, Laredo, Texas
The element radium can be found in extremely tiny amounts in the Earth’s crust and oceans, and in its pure form it is a soft silvery metal. To an untrained eye, a small piece of radium may look like a chip off a regular gray rock. But radium can invisibly emit radiation – energy and small fragments of itself – that you can’t feel, see or smell. And that invisible radiation can hurt you, without you even noticing right away.
What’s going on with this silent threat that can stealthily damage your body in ways that can take years to reveal themselves?
As a chemist, I’m interested in what makes different elements safe to handle or hazardous. This dangerous release of radiation is called radioactivity, and even though its source may look unassuming, it can burn you or even give you diseases that don’t manifest for years.
Atoms and isotopes
Everything you see around you – your skin, rocks, the pages of books – is all made up of different combinations of extremely small particles called atoms.
An atom has a small, dense center called the nucleus. Negatively charged particles called electrons move around the nucleus. Inside the nucleus, there are two types of particles: positively charged protons and neutral neutrons.
All atoms with the same number of protons in their nuclei are the same element. Besides radium, some elements you may have heard of are carbon and oxygen. All carbon atoms have six protons and all oxygen atoms have eight protons. Radium atoms are much heavier – all radium atoms have 88 protons.
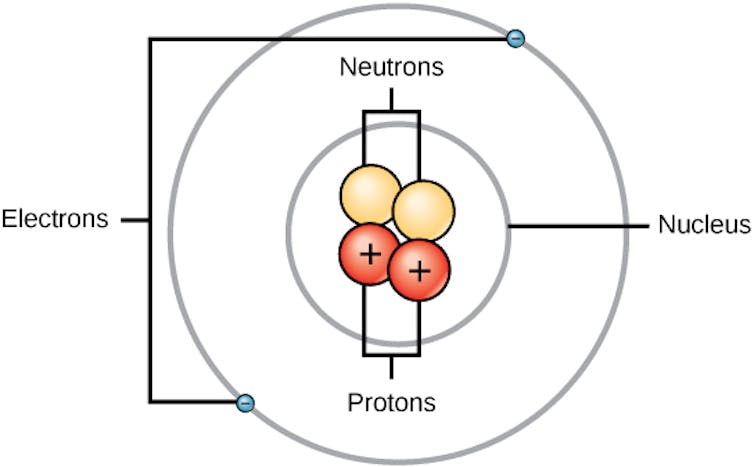
A simplified model of an atom, where the nucleus, containing neutrons and positively charged protons, sits in the center surrounded by negatively charged electrons.
CNX OpenStax/Wikimedia Commons, CC BY
Interestingly, it is possible for atoms of the same element to have different numbers of neutrons. Atoms of the same element with different numbers of neutrons are called isotopes. For instance, two carbon atoms would each have six protons, but one might have six neutrons while another could have seven or eight.
The number of protons and neutrons packed together in the nucleus determines whether the nucleus of an isotope is stable or not. If the nucleus is not stable, problems can arise.
Radioactive decay
The nucleus of each atom wants to be stable, but only certain arrangements of protons and neutrons make that possible. The number of protons and neutrons do not have to be equal, but some combinations make for a happy, or stable, coexistence in the nucleus while others don’t.
A nucleus with an unhappy mix of protons and neutrons might break down or deteriorate in some way. That process is called