Cell division, or the process of how daughter cells emerge from a mother cell, is fundamental to biology. Every cell inherits the same protein and DNA building blocks that make up the cell it originally came from. Yet exactly how these molecular building blocks arrange themselves into new cells has remained a mystery.
Studying cell division requires simultaneously viewing nanometer-scale macromolecules like proteins and DNA all the way up to millimeter-scale populations of cells, and over a time frame that ranges from seconds to weeks. Previous microscopes have been able to capture tiny objects only in short time frames, typically just tens of seconds. There hasn’t been a method that can examine a wide range of size and time scales all at once.
My team and I at the University of Michigan’s Bioplasmonics Group developed a new kind of superresolution imaging that reveals previously unknown features of how cells divide.

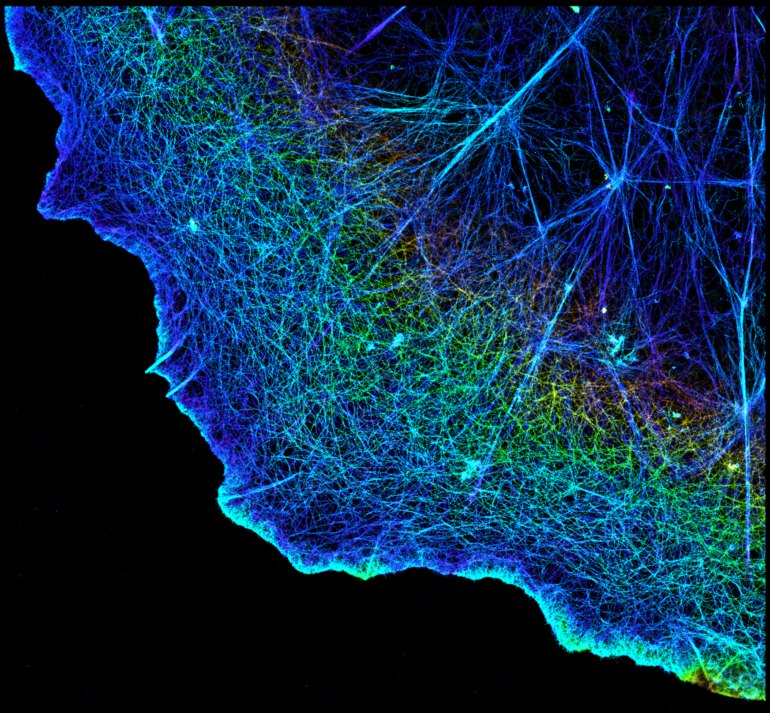
This hourglass depicts the process of superresolution over time, where the bottom shows a protein and the top a dividing cell going from unresolved, at left, to resolved, at right.
Somin Lee, CC BY-ND
Advancing superresolution imaging
It wasn’t possible to view cells at the molecular level until recently with the 2014 Nobel Prize-winning development of superresolution.
Traditional light microscopes blur very small objects that are close together in a sample, because light spreads out as it moves through space. With superresolution, fluorescent probes attached to the sample could be switched on and off like twinkling stars on a clear night. By collecting and combining many images of these probes, a superresolution image can bring very small objects into view. Superresolution opened a whole new world in biology, revealing structures as small as 10 nanometers, which is about the size of a protein molecule.
However, the fluorescent probes that this technique relies on can quickly wear out. This limits its use in studying processes that take place over extended periods, such as cell division.
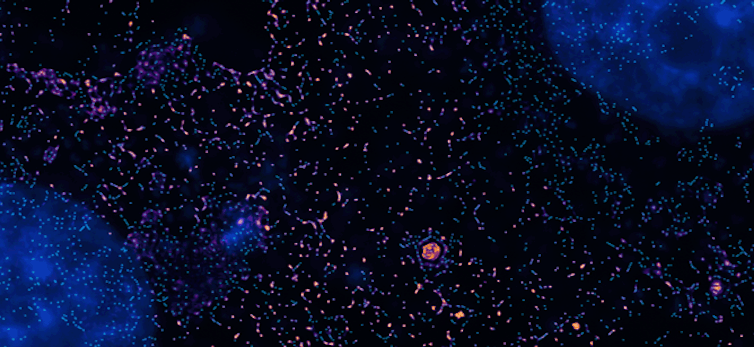
This PINE microscopy image shows cells dividing, their nuclei stained blue.
Somin Lee/Nature Communications, CC BY
My research team and I have a developed a solution we call PINE nanoscopy. Instead of absorbing light as traditional fluorescent probes do, the probes we use scatter the light so they do not break down with repeated light exposure.
To resolve very small objects that are close together, we built filters made of thin layers of polymers and liquid crystals that allow for detection of scattered light, which triggers the probes to switch on and off. This allowed us to see nanometer-scale details of cells that would otherwise be blurred by traditional microscopes.
Remarkably, we found that these nanometer-scale details could be viewed for very long periods – over 250 hours. These details would typically be lost over time with traditional…