Some asteroids have measured densities higher than those of any elements known to exist on Earth. This suggests that they are at least partly composed of unknown types of “ultradense” matter that cannot be studied by conventional physics.
Jan Rafelski and his team at the Department of Physics, The University of Arizona, Tucson, U.S., suggest that this could consist of superheavy elements with atomic number (Z) higher than the limit of the current periodic table.
They modeled the properties of such elements using the Thomas-Fermi model of atomic structure, concentrating particularly on a proposed “island of nuclear stability” at and around Z=164 and extending their method further to include more exotic types of ultra-dense material. This work has now been published in The European Physical Journal Plus.
Superheavy elements are defined as those with a very high number of protons (high atomic number), generally considered to be those with Z>104. They can be divided into two groups. Those with atomic numbers between 105 and 118 have been made experimentally but are radioactive and unstable with very short half-lives and, therefore, are only of academic and research interest.
Elements with Z>118 have not yet been observed, but properties have been predicted for some of them. In particular, an “island of nuclear stability” is predicted at about Z=164. And as, in general, the density of elements tends to rise with their atomic mass, these superheavy elements can be expected to be extremely dense.
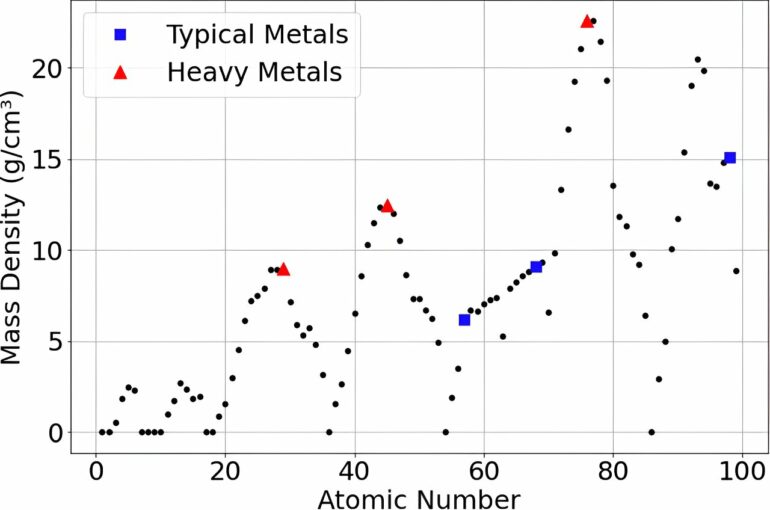
The densest stable element is the rare platinoid metal osmium (Z=76); its density of 22.59 g/cm3 is about twice that of lead. Objects—typically, astronomical bodies—with densities higher than that are considered “compact ultradense objects” or CUDOs.
The most extreme example known is the asteroid named 33 Polyhymnia, which is located in the main belt between Mars and Jupiter; its density has been calculated as about 75 g/cm3. Rafelski proposes that Polyhymnia and similar objects may be composed of elements above Z=118, possibly with other types of ultradense matter.
Rafelski and his two student co-workers, Evan LaForge and Will Price set out to calculate the microscopic atomic structure and properties of ultraheavy elements using the relativistic Thomas-Fermi model of the atom.
“We chose this model, despite its relative imprecision, because it allows the systematic exploration of atomic behavior as a function of atomic number beyond the known periodic table,” Rafelski explains. “A further consideration is that it also enabled us to explore many atoms in the short time available to Evan [LaForge], our brilliant undergraduate student.”
The researchers’ calculations confirmed the prediction that atoms with around 164 protons in their nuclei were likely to be stable, and, furthermore, suggested that a stable element with Z=164 would have a density between 36.0 and 68.4 g/cm3: a range that approaches the expected value for asteroid Polyhymnia.
As their model used the charge distribution in the atomic nucleus as one of its inputs, it could be extended to simulate still more exotic substances including alpha matter: a condensate composed entirely of isolated helium nuclei (alpha particles).
The idea that some asteroids may be composed of materials unknown on Earth is further motivating potential “space miners” who are planning to exploit the precious metals, including gold, that are expected to lie close to the surface of others.
“All super-heavy elements—those that are highly unstable as well as those that are simply unobserved—have been lumped together as ‘unobtainium,'” concludes Rafelski. “The idea that some of these might be stable enough to be obtained from within our solar system is an exciting one.”
More information:
Evan LaForge et al, Superheavy elements and ultradense matter, The European Physical Journal Plus (2023). DOI: 10.1140/epjp/s13360-023-04454-8
Citation:
Beyond the periodic table: Superheavy elements and ultradense asteroids (2023, October 10)