Scientists from MIT and the Indian Institute of Technology Madras have grown small amounts of self-organizing brain tissue, known as organoids, in a tiny 3-D-printed system that allows observation while they grow and develop. The work is reported in Biomicrofluidics.
Current technology for real-time observation of growing organoids involves the use of commercial culture dishes with many wells in a glass-bottomed plate placed under a microscope. The plates are costly and only compatible with specific microscopes. They do not allow for the flow or replenishment of a nutrient medium to the growing tissue.
Recent advances have used a technique known as microfluidics, where a nutrient medium is delivered through small tubes connected to a tiny platform or chip. These microfluidic devices are, however, expensive and challenging to manufacture.
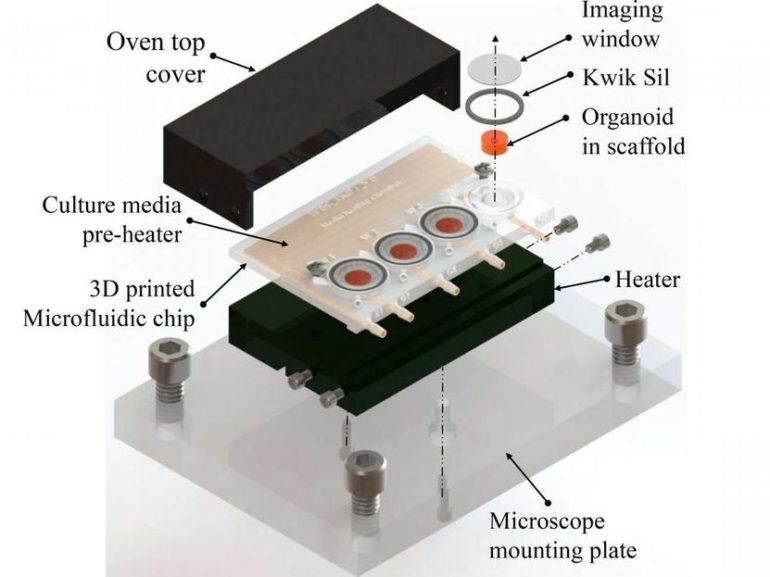
The current advance uses 3-D printing to create a reusable and easily adjustable platform that costs only about $5 per unit to fabricate. The design includes imaging wells for the growing organoids and microfluidic channels to provide a nutrient medium and preheating that supports tissue growth.
A biocompatible type of resin used in dental surgery was used for the 3-D-printed device. The printed chip was cured by exposing it to UV light, then sterilized before live cells were placed in the wells. After sealing the top of the wells with a glass slide, the nutrient medium and drugs for use in the study were added through small inlet ports.
“Our design costs are significantly lower than traditional petri dish- or spin-bioreactor-based organoid culture products,” said author Ikram Khan. “In addition, the chip can be washed with distilled water, dried, and autoclaved and is, therefore, reusable.”
The investigators tested their device with organoids derived from human cells. They observed the growing brain organoids with a microscope and were able to successfully follow their growth and development for seven days. The small bit of brain tissue developed a cavity or ventricle surrounded by a self-organizing structure that resembles a developing neocortex.
The percentage of cells in the core of the organoid that died during this one-week period was smaller in the 3-D-printed device than in regular culture conditions. The investigators believe that their cell design protects the tiny growing brain.
Khan said, “One advantage offered by our microfluidic device is that it allows constant perfusion of the culture chamber, which more closely mimics a physiological tissue perfusion than conventional culture, and thus reduces cell death at the organoid core.”
The investigators hope to increase the capacity of their device by scaling up the number of available wells. Other improvements will allow for additional instruments to be integrated into the design.
Flat brain organoids grown on 3D-printed scaffolds show intrinsic gyrification
More information:
“A low-cost 3D printed microfluidic bioreactor and imaging chamber for live-organoid imaging” Biomicrofluidics (2021). aip.scitation.org/doi/10.1063/5.0041027
Provided by
American Institute of Physics
Citation:
Tiny brains grown in 3D-printed bioreactor (2021, April 6)
retrieved 11 April 2021
from https://techxplore.com/news/2021-04-tiny-brains-grown-3d-printed-bioreactor.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.