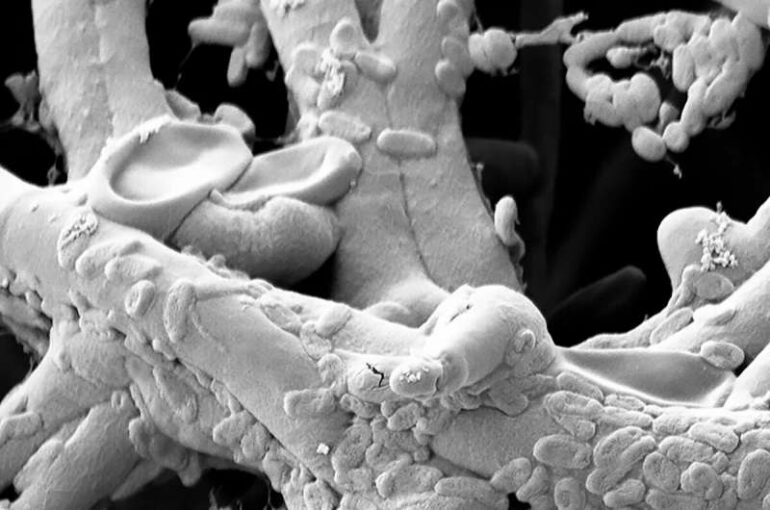
In nature, the roots of healthy plants are colonized by complex microbial communities of bacteria and filamentous eukaryotes (i.e., fungi and oomycetes, Fig. 1), the composition of which profoundly influences plant health. Maintaining a microbial equilibrium in their roots is very important for plants to remain healthy, however, the means by which this is achieved by plants is still largely unknown. Now, in a new study published in PNAS, Stéphane Hacquard and his colleagues from the Department of Plant-Microbe Interactions at the MPIPZ in Cologne, Germany, shed light on the host and microbial factors that are required to maintain a beneficial relationship between plant roots and their diverse microbial partners.
To tackle this research question, the first author of the study Katarzyna W. Wolinska used a complex microbial community comprising 183 bacteria (B), 25 fungi (F), and 6 oomycetes (O) that were isolated from roots of healthy Arabidopsis thaliana (Thale Cress) plants. She observed that this complex BFO community was beneficial for plant growth compared to sterile control plants grown in the absence of microbes. The authors then hypothesized that inactivation of specific components of the plant innate immune system—the system responsible for tackling pathogen infection—would result in an altered microbial equilibrium in roots, thereby affecting plant health. Consistent with this hypothesis, the beneficial BFO community was no longer beneficial in several of the immunocompromised mutant plants. In particular, inactivation of two plant host genes involved in tryptophan-derived specialized antimicrobials was sufficient to turn the beneficial BFO community into a detrimental community that negatively affected plant performance. The scientists then examined the presence of abnormal microbial signatures in the roots of these immunocompromised plants and found that the major factor that could explain growth differences across plants was the fungal load in their roots. This observation led to the conclusion that the fungal burden observed in the plant roots, in the absence of an intact immune system, was likely the primary cause explaining the shift from a healthy to an unhealthy state.
To further explore whether the presence of fungi in the plant root microbial community was indeed the direct cause of disease observed in the plants, K. W. Wolinska used the B, F, and O communities separately or in various combinations (BO, FO, BF, BFO) and observed that the presence of the fungi was indeed necessary to induce the unhealthy state of the plants. These results indicate that the production of specialized anti-fungal molecules from the host plant during tryptophan metabolism is key to maintain a healthy fungal equilibrium in plant roots. Interestingly, these anti-fungal molecules appeared to be insufficient in fully protecting plants from fungi in the absence of bacteria, even in the presence of a fully intact immune system.
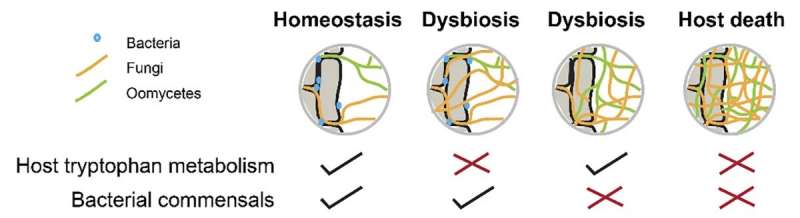
Figure. 2. Simplified model illustrating that the host tryptophan metabolism and the presence of bacterial commensals are both needed to protect plants from extensive colonization by filamentous eukaryotes. © Max Planck Society
According to the head of the study, Stéphane Hacquard, “Our results illustrate how host- and bacterium-encoded functions act in concert to maintain fungi in check in Arabidopsis roots, thereby promoting plant health and maintaining growth-promoting activities of multi-kingdom microbial communities. The observation that the protective activity of the bacterial community is as important as the host innate immune branch involving tryptophan-derived specialized metabolites for controlling fungi is remarkable. It indicates that the plant immune system is insufficient to fully protect plants from fungal burden, and that bacterial partners residing in roots provide an additional layer of protection, which is needed for plant survival.” (Fig. 2)
These findings have important applications for promoting plant health and turning potentially harmful fungi into beneficial isolates. By applying the knowledge gained in this study it would now be conceivable to design mixed bacterial-fungal synthetic communities that are expected to provide great fitness benefits to the host.
More information:
Katarzyna W. Wolinska et al, Tryptophan metabolism and bacterial commensals prevent fungal dysbiosis in Arabidopsis roots, Proceedings of the National Academy of Sciences (2021). DOI: 10.1073/pnas.2111521118
Provided by
Max Planck Society
Citation:
Host and resident bacteria join forces to control fungi in plant roots (2021, December 3)