Teaching the body’s immune cells to recognize and fight cancer is one of the holy grails in medicine. Over the past two decades, researchers have developed new immunotherapy drugs that stimulate a patient’s immune cells to significantly shrink or even eliminate tumors. These treatments often focus on increasing the cancer-killing ability of cytotoxic T cells. However, these treatments appear to only work for the small group of patients who already have T cells within their tumors. One 2019 study estimated that under 13% of cancer patients responded to immunotherapy.
To bring the benefits of immunotherapy to more patients, scientists have turned to synthetic biology, a new field of study that seeks to redesign nature with new and more useful functions. Researchers have been developing a novel type of therapy that directly gives patients a new set of T cells engineered to attack tumors: chimeric antigen receptor T cells, or CAR-T cells for short.
As an oncology physician and researcher, I believe that CAR-T cell therapy has the potential to transform cancer treatment. It’s already being used to treat lymphoma and multiple myeloma, and has shown remarkable response rates where other treatments have failed.
However, similar success against certain types of tumors such as lung or pancreatic cancer has been slower to develop because of the unique obstacles they put up against T cells. In our newly published research, my colleagues and I have found that adding a synthetic circuit to CAR-T cells could potentially help them bypass the barriers that tumors put up and enhance their ability to eliminate more types of cancer.
CAR-T cell therapy is currently only used for certain types of blood cancers.
How does CAR-T cell therapy work?
CAR-T cell therapy starts with doctors isolating a patient’s T cells from a sample of their blood. These T cells are then taken back to the lab, where they are genetically engineered to produce a chimeric antigen receptor, or CAR.
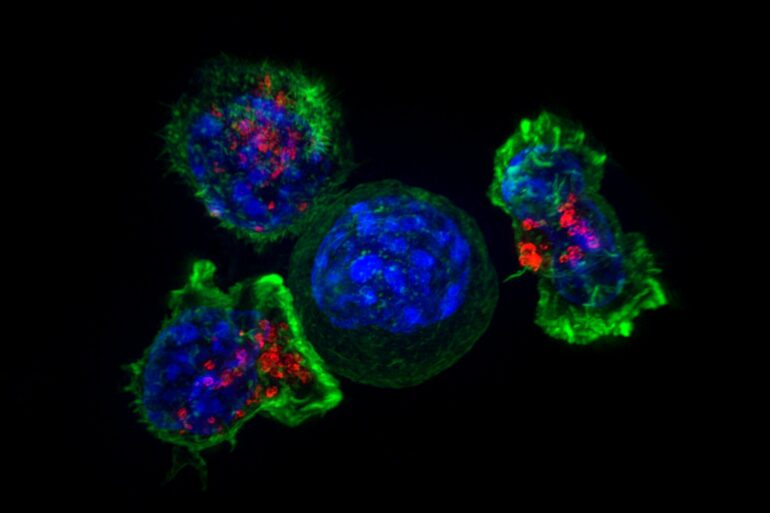
CARs are synthetic receptors specifically designed to redirect T cells from their usual targets have them recognize and hone in on tumor cells. On the outside of a CAR is a binder that allows the T cell to stick to tumor cells. Binding to a tumor cell activates the engineered T cell to kill and produce inflammatory cytokines proteins that support T cell growth and function and boost their cancer-killing abilities.
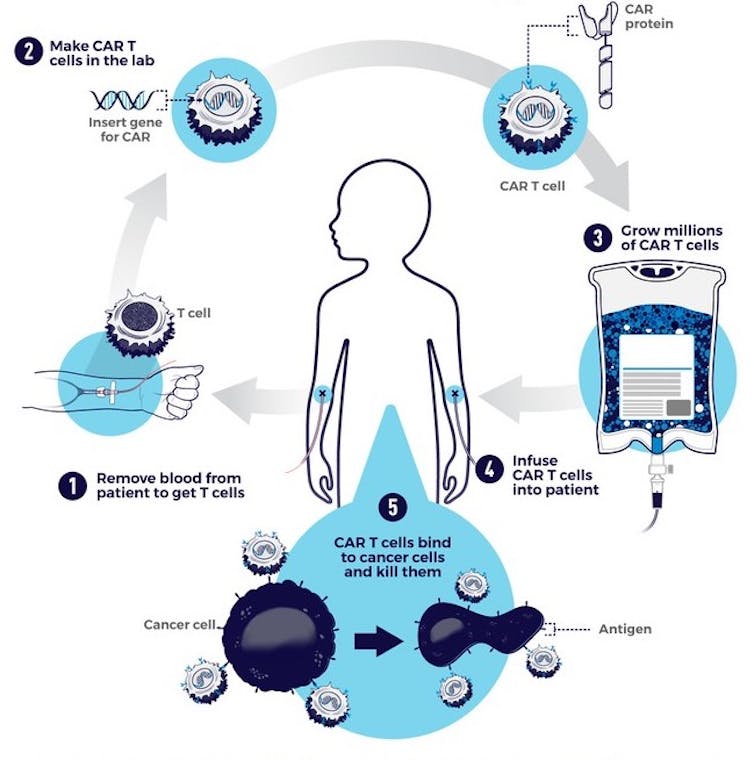
CAR-T therapy involves engineering a patient’s own T cells to attack their cancer.
National Cancer Institute (NCI)
These CAR-T cells are then stimulated to divide into large numbers over seven to 10 days, then given back to the patient via infusion. The infusion process usually takes place at a hospital where clinicians can monitor for signs of an overactive immune response against tumors, which can be deadly for the patient.