National University of Singapore researchers have developed an approach to selectively target pathogenic bacteria by harnessing an intracellular metabolite known as formate, abundant in these bacteria, as a new antimicrobial strategy. Formate is an essential metabolite needed for growth in certain pathogenic strains but is only found in low amounts in mammalian cells.
The abundance and evolving pathogenic behavior of bacterial microorganisms give rise to antibiotic tolerance and resistance, which pose a danger to global public health. New therapeutic strategies are therefore needed to keep pace with this growing threat.
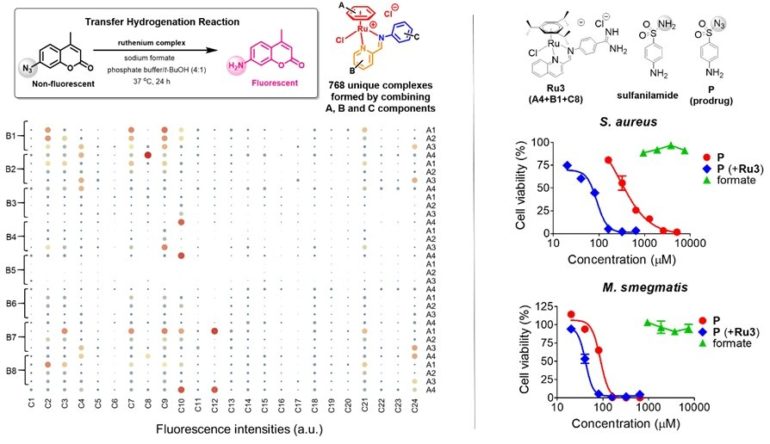
The research team led by Prof Ang Wee Han from the Department of Chemistry, NUS proposed a new approach to target bacteria by harnessing formate, a cell metabolite found only in particular bacterial species, to activate an antibacterial prodrug and selectively inhibit bacterial growth. The researchers developed a molecular caging system for sulfonamide antibacterial drugs by replacing their essential amide function group with azide (N3–). They also found an aqueous-stable compound (organoruthenium complex) that can release these antibacterial drugs under certain conditions after screening through 768 unique ruthenium complexes. When used together, the antibacterial drugs are released in the presence of endogenous formate found within bacterial cells. This strategy exploited formate, a necessary ingredient for bacterial growth, as a weapon to activate sulfonamide prodrugs using ruthenium complexes developed by the researchers.
Prof Ang said, “This is the first report that harnesses unique cellular metabolite to trigger prodrug activation in bacteria. It paves the way for a new approach of targeted antibacterial therapy by exploiting differences in the occurrence of natural metabolites among pathogenic strains.”
The rationally designed sulfonamide prodrug with azide caging moiety was efficiently activated by the organoruthenium complexes in formate-abundant bacteria with up to 8-fold enhancement in drug efficacies, particularly in the pathogenic bacteria, Staphylococcus aureus and Escherichia coli. In contrast, efficacy elevation in Mycobacterium smegmatis, which has limited formate generation capacity, increased only by two-fold. This validated the thesis that intracellular metabolite formate can be targeted for the development of novel antibacterial therapeutic strategies.
Prof Ang said, “Antibiotic resistance is one of the most serious challenges confronting humanity and yet development of new antibiotics has not kept pace with this growing threat. The unique bacterial metabolome offers new possibilities for addressing this issue. We hope that this discovery will spur new research targeting distinct metabolites for the development of better and more selective antibacterial agents.”
The research team is working toward developing other prodrug activation strategies that can harness endogenous bacterial formate. In addition, investigations into other clinical antibiotics such as trimethoprim and sulfamethoxazole are also in progress.
A plant defense metabolite specifically suppresses virulence of pathogenic bacteria
More information:
Cheng Weng et al. Harnessing Endogenous Formate for Antibacterial Prodrug Activation by in cellulo Ruthenium‐Mediated Transfer Hydrogenation Reaction, Angewandte Chemie International Edition (2020). DOI: 10.1002/anie.202000173
Provided by
National University of Singapore
Citation:
Antibacterial prodrug by targeting intracellular metabolite (2020, October 9)
retrieved 11 October 2020
from https://phys.org/news/2020-10-antibacterial-prodrug-intracellular-metabolite.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.