All biological processes in our cells are constantly monitored to prevent the accumulation of defective proteins. In the worst case, such protein clumps can trigger diseases. The synthesis of new proteins is particularly susceptible to errors. Erronous proteins must then be removed by our cells. Until now, it was unclear how exactly this process works.
Researchers led by F.-Ulrich Hartl at the MPIB now discovered a new mechanism that can initiate the targeted degradation of defective proteins. The protein GCN1 is of crucial importance in this process. The results have been published in the journal Cell.
Ribosomes are molecular machines that produce all proteins in our cells. The genetic code of an organism is transcribed into the so-called messenger RNA, or mRNA for short. Ribosomes read these blueprints to translate them into a wide variety of proteins. They carefully stitch amino acids together, until a long chain is formed which then is folded into a functional protein.
However, errors may arise during this process, as nothing in life is exempt from mistakes. For example, ribosomes may run past the STOP signal in the blueprint and assemble more amino acids than required. Such erroneous proteins can be non-functional or even worse, these defective proteins can accumulate to form protein clumps, a hallmark of various neurodegenerative diseases, such as Alzheimer’s or Parkinson’s disease.
Previous studies discovered that cells have the remarkable ability to recognize such defective proteins and also to eliminate them. However, the exact mechanism remained unknown. To decipher the underlying clearance pathway, the researchers used the worm model organism C. elegans, as well as human cells.
The firefighter protein calls for degradation
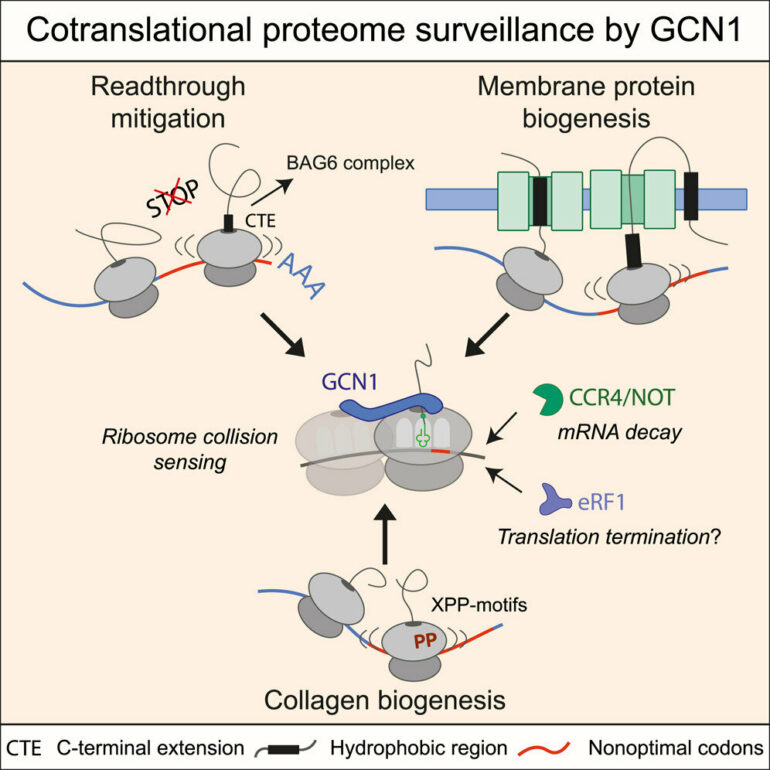
Upon closer examination of how defective proteins are eliminated, the scientists unexpectedly discovered that the mRNA itself is also degraded. They suspected that the problematic mRNA is already recognized at the ribosome during translation. In this context, the researchers found a complex that was already known to play a role in the degradation of mRNAs. In addition, they discovered that the GCN1 protein plays an important role to initiate this process.
Like many cars on a road, several ribosomes traverse a mRNA at the same time to translate the blueprint into proteins. Sometimes, ribosomes like two cars which are following each other, can collide if the first car brakes unexpectedly, for example, because a cat jumps onto the road. The GCN1 protein then acts like a firefighter who is at the scene of the accident as a first responder. It stabilizes and secures the accident site to then call the towing service and road cleaning service, which remove the collided vehicles and also renew the road surface if necessary.
The complexes in our cells that are called by the firefighter protein break down the problematic mRNA. But how exactly does the protein recognize that an accident has occurred and that towing service and road cleaning are needed?
Profiling of the firefighter protein
Crucial insights were gained using a technique called selective ribosome profiling (SeRP), which makes it possible to determine the exact location of ribosomes on mRNAs. The researchers looked for where all the ribosomes bound to a firefighter protein were located, regardless of whether they were still driving or already were involved in a collision. They found that the firefighter protein intervenes when a ribosome has produced a chain of amino acids that is too long and has overshot its actual STOP signal in the process. Since in this situation there is an increased number of collisions between two ribosomes, the firefighter protein then calls for accident cleanup.
In addition, the scientists found that the GCN1 protein is not only involved in monitoring overrun STOP signals. In particular, GCN1 was enriched on ribosomes that translate membrane proteins and collagens encoding mRNAs. Deeper analysis revealed that a common feature, that makes these three classes firefighter targets, are so-called non-optimal codons, a sequence of nucleotides on the genome that function like a speed limit on the road. In addition, they found that stabilization of the ribosome accident by the firefighter protein GCN1 also calls molecular chaperones to the scene of the accident. Chaperones are a class of proteins that help other proteins to fold correctly.
The firefighter protein supports the healthy aging of our cells
Aging is a risk factor for various diseases. Defective proteins become more common with increasing age and pose a threat to the health of an organism. It was shown that a malfunction of the firefighter protein can shorten the life expectancy of the model organism C. elegans. In fact, such a malfunction caused more proteins to accumulate and cluster together in older worms, which may promotes neurodegenerative diseases.
In the experiments with human cell lines, the researchers were able to show that impairments in the management of protein balance also occur here. With the results of the study, the scientists hope to find ways in the future to reduce the age-related accumulation of defective proteins in order to prevent neurodegenerative diseases such as Alzheimer’s or Parkinson’s diseases.
More information:
Martin B.D. Müller et al, Mechanisms of readthrough mitigation reveal principles of GCN1-mediated translational quality control, Cell (2023). DOI: 10.1016/j.cell.2023.05.035
Provided by
Max Planck Society
Citation:
Discovery of a new mechanism that may promote healthy aging of our cells (2023, June 20)