WEHI researchers have produced the first molecular images of an enzyme that controls proteins to signal and communicate with each other in human cells. The discovery could help to solve the mystery cause of a rare group of hereditary neurodegenerative diseases linked to deregulation of this enzyme.
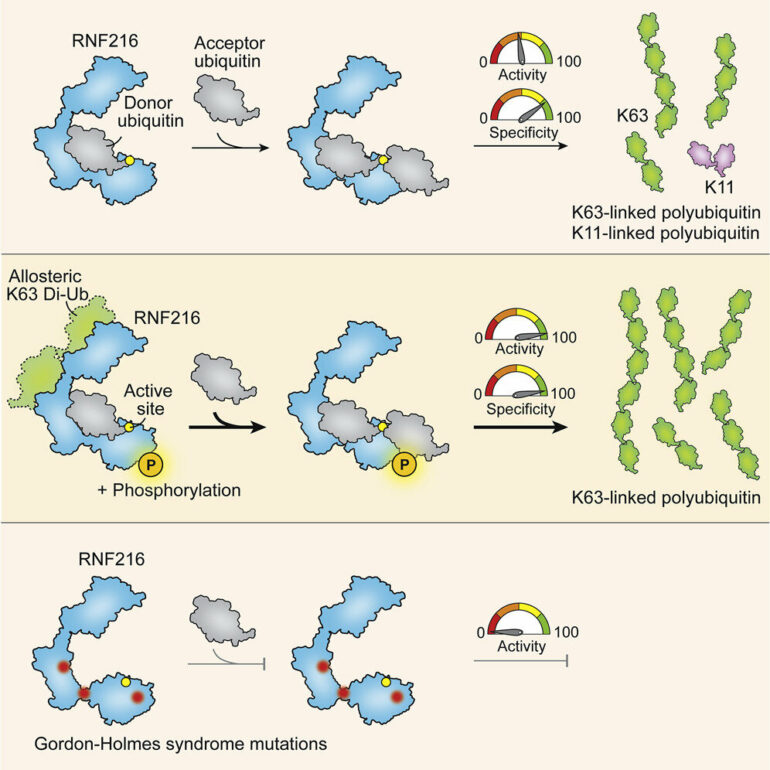
In the study, published in Molecular Cell, Dr. Thomas Cotton, Dr. Bernhard Lechtenberg and colleagues at WEHI solved the first three-dimensional (3D) structure of an enzyme called RNF216. The team captured molecular ‘snapshots’ of RNF216 as it assembled chains of the small protein ubiquitin, which tags the target proteins to modify their behavior in the cell. They also showed how RNF216 dictates the type of ubiquitin chain that is formed—the first time that this has been explained.
Mutations in RNF216 have been linked to Gordon-Holmes Syndrome, a very rare neurodegenerative disorder that results in reproductive problems, movement disorders, and progressive cognitive decline and dementia.
Caught in the act
Ubiquitin is a small protein that is found in every cell of the body. Ubiquitin ligases attach chains of ubiquitin to other target proteins in the cell. The specific type and nature of the ubiquitin chain influences the target protein’s behavior; some ubiquitin chains direct the protein to be destroyed, while others tell the protein to relocate within the cell, or facilitate protein-protein signaling.
Dr. Lechtenberg said ubiquitin chains were instructions that informed how the protein should behave.
“Ubiquitination of proteins essentially regulates every aspect of behavior of a cell in the human body. Understanding how different chain types are formed by ubiquitin ligases is critically important and—in the long term—could enable us to develop drugs that influence cellular behavior,” he said.
In this paper, his team studied RNF216, a ubiquitin ligase that was known to assemble K63-linked ubiquitin chains.
“Ubiquitin ligases are enzymes that chemically link ubiquitin to target proteins. K63-linked chains are responsible for signal transmission or communication between proteins within cells,” Dr. Lechtenberg said.
“In this paper, we captured the first ever image of RNF216, and we also caught it in the act of forming these K63-linked ubiquitin chains. We were able to develop a molecular blueprint of the protein at several key stages to reveal the biochemical reactions that enable it to recruit ubiquitin and form these chains.”
Changing behavior
Dr. Cotton said the team also showed, for the first time, how another enzyme ‘supercharged’ RNF216 activity to greatly increase ubiquitin chain production and ensure it only produces K63-linked chains.
“After we solved the crystal structures using the Synchrotron, we worked with our colleagues in WEHI’s proteomics facility to study what types of ubiquitin chains were formed,” he said.
“We found something really interesting. Another regulatory enzyme ‘flicks a switch’ on the RNF216 protein that encourages it to preferentially form K63 links; in fact, it almost exclusively forms these types of links. So, the action of the regulatory enzyme not only increases binding of ubiquitin to RNF216 and efficiency of chain formation, it also directs the type of ubiquitin links that are made—a sort of molecular ‘finetuning’ that improves the specificity or ‘quality’ of the chain.
“We had no idea that this was happening, it is probably the most interesting finding for me,” Dr. Cotton said.
Neurological disease mystery
The human genome encodes around 600-700 ubiquitin ligases and an increasing number of genetic diseases have been linked to deregulation of ubiquitin ligases including RNF216, Dr. Lechtenberg said.
“We still don’t know exactly which target proteins RNF216 attaches ubiquitin chains to, or what the biological outcomes are. There is evidence that it might relate to innate immune signaling, such as early responses to virus and bacterial infection, as well as communication between neurons in the brain,” he said.
He added there were a small group of very rare, hereditary neurodegenerative disorders—Gordon-Holmes Syndrome (GHS) and Huntington’s-like diseases—that were linked to mutations in RNF216.
“GHS was first described more than 100 years ago and is associated with reproductive and neurological symptoms including movement disorders, progressive cognitive decline and early-onset dementia. However, it wasn’t until the past 10 years that US researchers studied the genomes of people with this rare disease and discovered mutations in RNF216,” Dr. Lechtenberg said.
He said the research team recreated mutations in the laboratory that mimicked the genetic mutations found in people with GHS.
“We were able to visualize the location of some of these important mutations and show that the mutations disrupt or block formation of the K63-linked ubiquitin chains,” he said.
“Now that we have pictures of the protein in action, we hope to be able to look at the healthy and mutated forms of the protein in cells to understand their biological function in more detail. That is the next step in our research.”
More information:
Thomas R. Cotton et al, Structural basis of K63-ubiquitin chain formation by the Gordon-Holmes syndrome RBR E3 ubiquitin ligase RNF216, Molecular Cell (2022). DOI: 10.1016/j.molcel.2021.12.005
Provided by
Walter and Eliza Hall Institute of Medical Research
Citation:
First images of enzyme provide insights into cause of hereditary neurological disease (2022, January 10)