Interdisciplinary scientific collaboration between two research Institutes of the Foundation for Research and Technology—Hellas (FORTH), the Institute of Molecular Biology and Biotechnology (IMBB) in Crete, and the Institute of Chemical Engineering Sciences (ICE-HT) in Patras, sheds light onto the interplay between metabolism and the rate of aging. The findings are published today in Nature Communications.
Metabolism is a complex network of biochemical reactions that allows organisms to utilize energy obtained through feeding, to grow and sustain a healthy life. Mitochondria are the main organelles for cellular energy conversions in all eukaryotic cells, and the site where the final steps of glucose breakdown take place. Importantly, alterations in metabolism critically affect the aging process. The study, published today, reveals that genetic or pharmacological manipulations of mitochondrial abundance in specific tissues modulate the rate of aging.
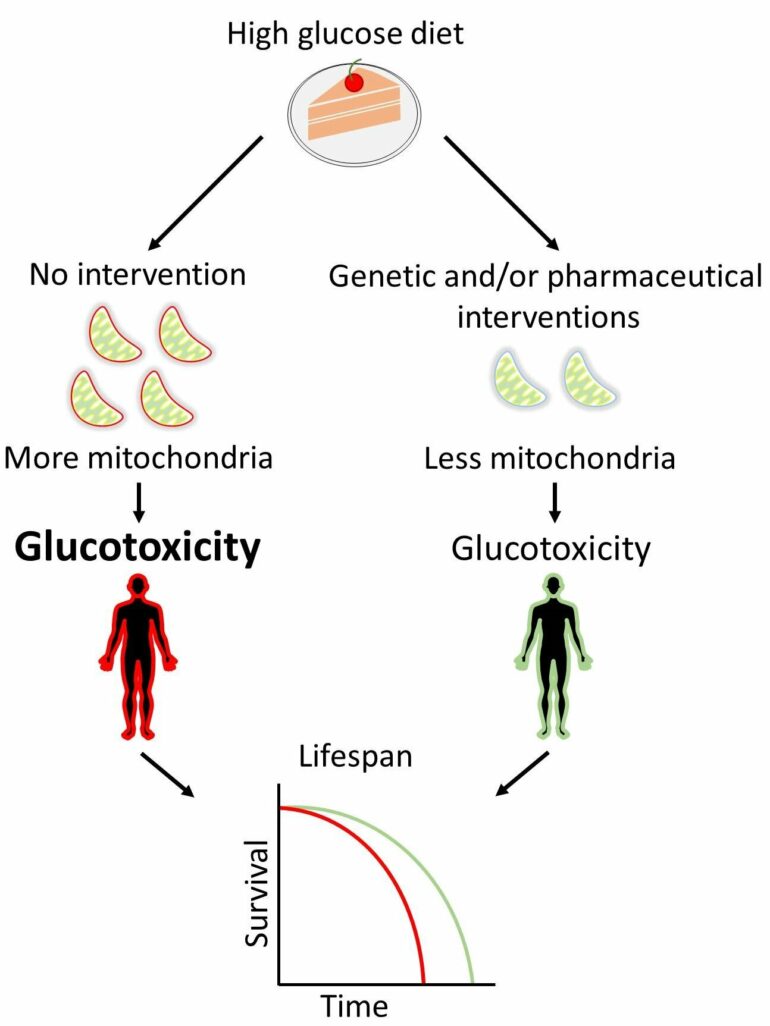
Using the nematode Caenorhabditis elegans, FORTH researchers now show that reduced mitochondrial abundance leads to extension of animal lifespan. Reduced mitochondrial abundance correlates with reduced reactive oxygen species production, induction of mitochondrial proteotoxic stress response mechanisms and induction of glucose uptake. Metabolic profiling of animals with reduced mitochondrial load highlights the rewiring of cellular glucose metabolism towards de novo biosynthesis of the amino acid serine. Interestingly, inhibition of serine production annuls the benefit that mitochondrial depletion exerts on lifespan, indicating a causative role of this biochemical pathway to longevity.
Prolonged exposure to high glucose levels is known to provoke the onset of metabolic diseases, such as diabetes and obesity. Indeed, FORTH Scientists found that increased dietary glucose uptake throughout development and adulthood critically compromises lifespan. Notably, reduction of mitochondrial load ameliorates glucotoxicity and improves health during old age.
These new findings provide a new framework for elucidating the mechanism whereby metabolic reprogramming contributes to longevity, and pave the way for designing tissue-specific interventions, aiming to alleviate pathologies associated with metabolic disorders.
More information:
Eirini Lionaki et al, Mitochondrial protein import determines lifespan through metabolic reprogramming and de novo serine biosynthesis, Nature Communications (2022). DOI: 10.1038/s41467-022-28272-1
Provided by
Foundation for Research and Technology – Hellas
Citation:
How metabolic reprogramming in mitochondria promotes or undermines survival and longevity (2022, February 4)