A research team led by the Hong Kong University of Science and Technology (HKUST) recently revealed how TMED10, a type of transmembrane protein, regulates muscle stem cell differentiation through mediating the secretion of insulin-like growth factor 2 (IGF2). This provides potential therapeutic strategies to downregulate IGF2 signaling by inhibiting its secretion.
IGF2 is a pivotal player in cellular processes such as proliferation, migration, differentiation, and survival. Its dysregulation has been linked to multiple growth disorders, including Silver–Russell syndrome and Beckwith–Wiedemann syndrome.
While the expression of IGF2 and its induced signal transduction pathway have been widely studied, the mechanisms through which newly synthesized IGF2 proteins are secreted to perform their functions have remained enigmatic.
Now, a research team led by Prof. Guo Yusong, Associate Professor of the Division of Life Science at HKUST, has provided valuable insights into this process. The study was a collaboration between research at HKUST and Hong Kong Polytechnic University (PolyU), and was published in Proceedings of the National Academy of Sciences (PNAS).
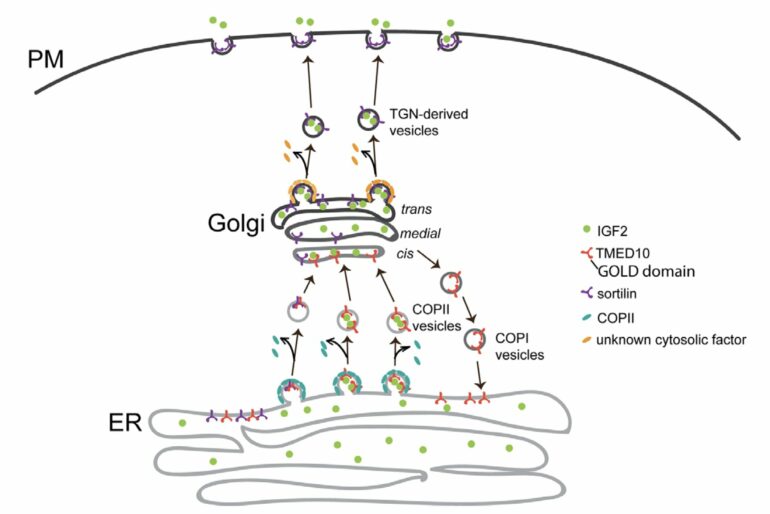
Newly synthesized IGF2 needs to pass through several intracellular transport stations, including the endoplasmic reticulum (ER) and the Golgi before it is secreted out of the cells. The study identified TMED10, a type I transmembrane protein, that facilitates the ER-to-Golgi export of IGF2 by recognizing an export motif on IGF2.
Further investigation revealed that this regulation is a result of direct interaction between the Golgi-dynamics (GOLD) domain of TMED10 and the residues 112-140 of IGF2. Additionally, mass spectrometry analysis showed that TMED10 also mediates the ER export of sortilin, a single-pass transmembrane protein from the vacuolar protein sorting 10 protein (Vps10p) family.
Subsequent studies suggested that sortilin aids in the post-Golgi trafficking of IGF2, implying that TMED10 indirectly mediates the trans-Golgi network (TGN) export of IGF2. The team also validated their model in mouse C2C12 myoblasts, demonstrating that TMED10 regulates muscle stem cell differentiation in an autocrine manner.
“These findings enhance our understanding of the physiological roles, disease mechanisms, and potential therapeutic applications of IGF2. In certain conditions, overexpression of IGF2 can trigger uncontrolled cell growth and potentially lead to cancer. By manipulating the trafficking processes, we may be able to modulate IGF2 signaling for therapeutic benefits,” said Prof. Guo.
“Furthermore, since IGF2 plays a critical role in tissue repair and regeneration, enhancing its release by overexpressing its cargo receptor in vivo could expedite wound healing.”
The research team also consists of Prof. Wu Zhenguo, Professor of HKUST’s Division of Life Science; and Prof. Yao Zhongping, Professor of PolyU’s Department of Applied Biology and Chemical Technology; and their team members.
More information:
Tiantian Li et al, TMED10 mediates the trafficking of insulin-like growth factor 2 along the secretory pathway for myoblast differentiation, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2215285120
Provided by
Hong Kong University of Science and Technology
Citation:
Researchers elucidate how IGF2’s secretory pathway mediates muscle stem cell differentiation (2023, December 8)