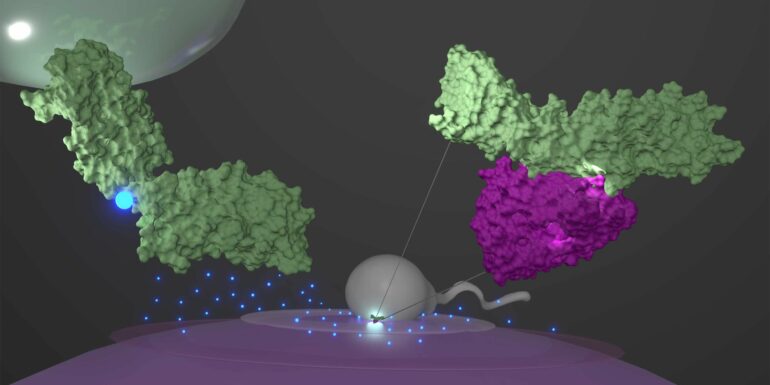
Researchers at ETH Zurich recently developed highly realistic simulations of the proteins on sperm and egg cells coupling together before they fuse. These findings enabled the research team to solve several mysteries of fertilization at once, which could help to accelerate development of more targeted infertility treatments.
Penetration of an egg cell by a sperm cell is a fundamental step in procreation, happening dynamically and seemingly without problems. However, if you zoom in on the processes that take place during fertilization at a molecular level, it becomes highly complex, and it is thus not surprising that 15% of couples worldwide struggle to conceive.
No microscope, however modern, can illuminate the countless interactions between the proteins involved. Therefore, the exact trigger for the fertilization process and the molecular events that transpire just before the fusion of the sperm and egg have remained murky—until now.
With the help of simulations on Piz Daint, the supercomputer of the Swiss National Supercomputing Center (CSCS), a research team led by ETH Zurich Professor, Viola Vogel has now made the dynamics of these crucial processes in the fertilization of a human egg cell visible for the first time. According to their study, which was recently published in the journal Scientific Reports, the researchers’ simulations have succeeded in revealing important secrets.
Special protein complex enables the fusion process
It was previously known that the first specific physical connection between the two germ cells is an interaction of two proteins: The JUNO, which is located on the outer membrane of the female egg cell, and the IZUMO1 on the surface of the male sperm cell.
“It was assumed that the combination of the two proteins into a complex initiates the recognition and adhesion process between the germ cells, thereby enabling their fusion,” says Paulina Pacak, a postdoctoral researcher in Vogel’s group and first author of the study. However, based on the crystal structure scientists had not yet been able to clearly describe the mechanism.
The ETH research team finally succeeded in doing this in their latest simulations. In order to create a realistic environment in the in-silico experiment, the researchers needed to simulate JUNO and IZUMO1 in an aqueous solution. In water, however, the protein moves, and the interactions with the water molecules change both the way the proteins bind to each other and, in some cases, the function of the proteins themselves.
“This makes the simulations much more complex, also because water alone already has a highly complex structure,” says Vogel, “but the simulations provide a more detailed picture of the dynamic of the interactions.”
The simulations on Piz Daint spanned 200 nanoseconds each and showed that the JUNO-IZUMO1 complex is stabilized by a network of more than 30 short-lived contacts—the individual bonds lasted less than 50 nanoseconds each.
According to the researchers, a deeper understanding of these network dynamics of the rapidly changing formation and breaking of individual bonds presents new possibilities for the development of contraceptives, as well as for better understanding mutations that affect fertility.
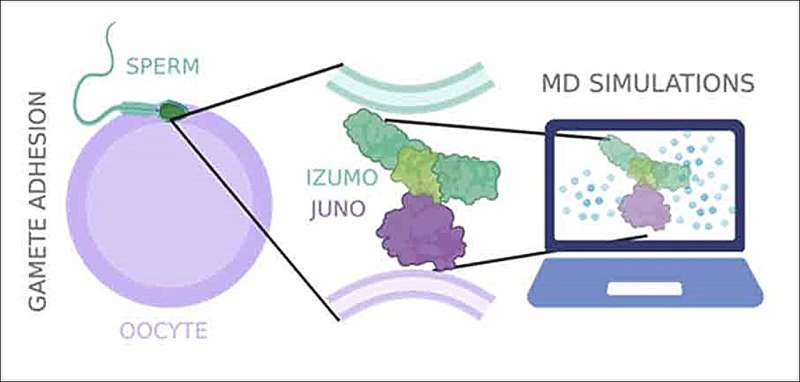
From the petri dish to in silico experiments: With the help of high-resolution simulations, the team led by ETH Professor Viola Vogel succeeded in visualizing what must happen between the two proteins JUNO and IZUMO1 on the egg (Oocyte) and sperm surfaces to induce the fertilization. © Research Group of Viola Vogel / ETH Zurich
Zinc ions regulate bond strength
With these network dynamics brought to light, the researchers then investigated how these vital protein binding could be destabilized. Zinc ions (Zn2+) play an important role here: If they are present, IZUMO1 bends into a boomerang-like structure as shown by the simulations and, as a result, IZUMO1 can no longer firmly bind to the JUNO protein.
According to the researchers, this could be one reason why the egg cell releases many zinc ions immediately after fertilization in a so-called “zinc spark.” This flood of zinc is known to prevent further sperm from penetrating the egg cell which would otherwise cause aberrant development.
“We can only find out something like this with the help of simulations. The findings that we derive from them would hardly be possible on the basis of the static crystal structures of the proteins,” emphasizes Vogel. “The highly dynamic process of fertilization takes place far away from the equilibrium. As available protein structures show them embedded in the crystal, resources such as those at CSCS are essential to capture and understand these interaction dynamics.”
Folic acid binding by IZUMO1
Thanks to the simulations, the researchers were able to unravel another mystery too: How naturally occurring folates and their synthetic equivalents, folic acids, bind to the JUNO protein. Expectant mothers are generally recommended to take folic acid supplements before a planned pregnancy and during the first three months to support healthy neural development in the fetus.
However, laboratory experiments have shown that the JUNO protein does not bind with folate in aqueous solution, even though JUNO itself is a folate receptor. The molecular dynamics simulations have now shown that Folate binding is possible once IZUMO1 binds to JUNO. Only then can the folate enter the presumed folate-binding pocket of JUNO.
These new discoveries are not only of fundamental interest for structural biology. They also provide a detailed basis for the development of active pharmaceutical ingredients.
According to the researchers, the decoded dynamic mechanisms of the interaction between the JUNO and IZUMO1 proteins could point to new ways of treating infertility, developing drug-based non-hormonal contraceptive methods, and improving in vitro fertilization technology.
More information:
Paulina Pacak et al, Molecular dynamics of JUNO-IZUMO1 complexation suggests biologically relevant mechanisms in fertilization, Scientific Reports (2023). DOI: 10.1038/s41598-023-46835-0
Citation:
Scientists successfully simulate protein complex that initiates fertilization (2024, February 2)