Each simple RNA virus has a genome, its “native RNA.” This genome dictates how the virus replicates in cells to eventually cause disease. The genome also has the code for making a capsid, the protein shell of a virus that encapsulates the genome and protects it like a nanocontainer.
A team led by Roya Zandi, a professor of physics and astronomy at the University of California, Riverside, has developed a theory and performed a series of simulations that may help explain how a virus finds its native genome and how capsids form around it and not around other RNAs in the cell.
“A better understanding of how capsids form is of vital importance to material scientists and a crucial step in the design of engineered nano-shells that could serve as vehicles for delivering drugs to specific targets in the body,” Zandi said.
The researchers’ work, published in ACS Nano, shows that the interplay of the mechanical properties of proteins, the size of the genome, and the strength of the interaction between the genome and capsid proteins can significantly modify the symmetry, structure, and stability of the capsid.
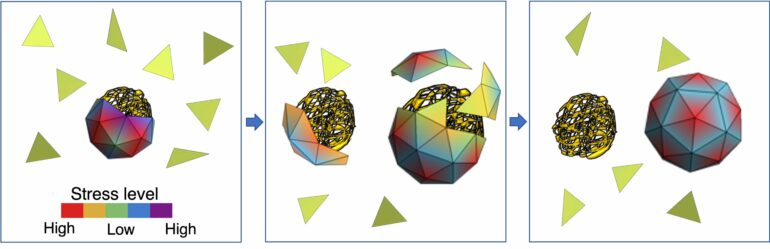
When a virus enters a cell, the capsid breaks open to release the genome, which then uses the cell’s reproductive machinery to replicate. The newly formed genomes begin to acquire their capsids, a process mainly driven by the attractive electrostatic interaction between the positive charges on capsid proteins and the negative charges on the genomes. But how the virus selects and packages its native RNA inside the crowded environment of a host cell cytoplasm in the presence of many nonviral RNA and other polymers has remained a mystery.
The simulations run by Zandi’s team show that capsid proteins could, in theory, pick any nonviral genome to encapsulate. But the viral genome, she said, is best suited for capsid proteins to form a shell around due to an interplay of energies at the molecular level.
“The stress distribution of the capsid proteins is lower when the capsids encapsulate their own genome, the one for which they were coded,” Zandi said. “The energy of the whole system is lower. While smaller nonviral RNAs are available in the cell in plenty, the capsid proteins are inclined toward forming a shell around a viral RNA because the resulting soccer ball-like shell has a lower stress distribution.”
Zandi said the work lays out a systematic comparison of theory and experiments, which will allow a better understanding of the role of RNA in the capsid assembly pathway, stability, and structure.
“A deeper understanding of the role of the genome in virus assembly mechanisms could lead to design principles for alternative antivirals,” she said.
The new work is an early step in understanding viral assembly. The process is not well understood because viruses measure in nanometers and the assembly occurs in milliseconds.
“Theoretical work and simulations are necessary to understand how a virus grows,” Zandi said.
Zandi was joined in the research by graduate student Sanaz Panahandeh at UCR; Siyu Li at Songshan Lake Materials Laboratory in China; and Bogdan Dragnea at Indiana University, Bloomington. Li is a former graduate student at UCR.
More information:
Sanaz Panahandeh et al, Virus Assembly Pathways Inside a Host Cell, ACS Nano (2022). DOI: 10.1021/acsnano.1c06335
Provided by
University of California – Riverside
Citation:
Simulations on how a virus packages its genetic material could help design nanocontainers used in drug delivery (2022, March 9)