During embryonic development, two different cascades of genetic signals determine whether the embryo’s primordial gonad will become testes or ovaries, and thus whether the embryo will develop into a male or a female. Disruptions in this process cause disorders in sexual development characterized by a mismatch between sex-determining chromosomes, gonads (ovaries or testicles) and the anatomy of the genitals. The incompatibility can be expressed in many and varied forms, such as unclear genitalia or a combination of male and female physiological characteristics. This medical condition is termed Disorders of Sex Development (DSD) with a prevalence of 1 in 4,500 newborns.
One of the significant challenges in sex reversal research is the lack of an in vitro system to model and study variants found in DSD individuals. A study published today by researchers at Bar-Ilan University offers a solution to this challenge through the development of new tools to create the somatic/supporting cells of the gonad and hence be able to model stem cells derived from a DSD individual in a dish. This facilitates, for the first time, the ability to begin investigating DSD pathologies in the lab dish in a human-related context (and not via mice models).
The research, led by Dr. Nitzan Gonen, of Bar-Ilan University’s Goodman Faculty of Life Sciences and Institute of Nanotechnology and Advanced Materials, in collaboration with The Francis Crick Institute in the UK and Institut Pasteur in France, was published in the journal Science Advances.
Approximately one year ago, an article by Yoshino et al. in the journal Science showed that stem cells can be used to make both egg progenitor cells and ovarian somatic cells. When the researchers combined these two cells together, they were able to create a healthy and fertile embryo as a product of these artificial eggs. Dr. Gonen’s research demonstrates the beginning stage of a similar idea, but this time in male cells.
Male testicles contain germ cells that develop into sperm cells starting from puberty and throughout life. These cells are surrounded by supporting somatic (non-germ) cells that allow germ cells to function and develop.
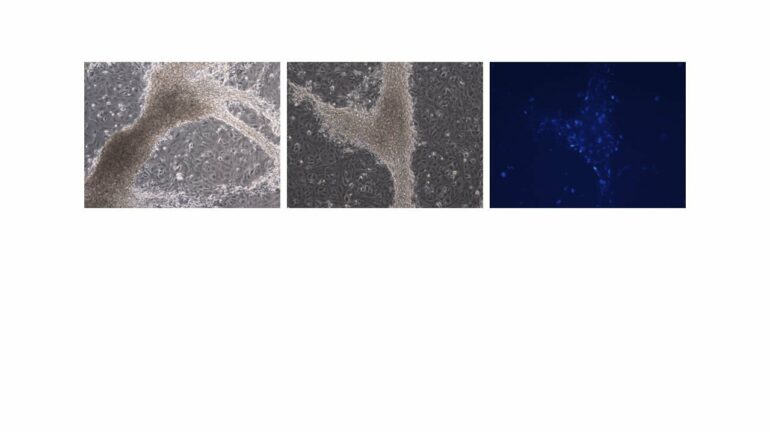
In the current study, Dr. Gonen and the researchers from The Francis Crick Institute differentiated mouse embryonic stem cells into early somatic cells in the testes. They compared the cells they created to real testis cells and showed, through RNA sequencing technology, that the two are very similar. The advantage of using mouse-derived stem cells first is that it enables proper comparison to real gonadal cells, isolated from embryonic gonads. It is very difficult—even impossible—to do this with human cells and human embryos, because the human equivalent is an embryo at week seven of pregnancy, a stage where access to embryos following abortions is challenging.
Dr. Gonen’s partners from Pasteur Institute, Dr. Anu Bashamboo and her lab, took this and showed that it works on human stem cells in a very similar way. Different combinations of X and Y chromosomes determine the sex of the embryo. The researchers took three types of human cells—cells from a male (XY), cells from a female (XX) and cells from a sex-reversed DSD individual (XY born as female). They showed that the somatic cells produced from XX and XY are different from each other, while the cells of the sex-reversed individual are somewhere in the middle, closer to a female than to a male. However, when the variant or mutation in the DSD individual cells was corrected with CRISPR genome editing technology, the cells returned to behaving like typical XY cells.
The creation of this cellular model of a human with sex reversal opens the door to understanding where the sex determination process went wrong in many other unexplained cases of DSD and what exactly is altered in the DSD individual’s cells.
“I think this study presents the possibility to generate various different somatic cell types of the gonad like Sertoli cells which support germ cells, or Leydig cells which secrete testosterone,” says Dr. Gonen. “Combining somatic supporting cells with germ cells will enable us to create a “mini testis in a dish” to better understand cases of DSD and infertility. Hopefully, we may be able to use that in the future to generate functional sperm in the lab to allow infertile men to have a biological child.”
“Being able to understand the reasons behind differences in sex development is often very valuable to the individual affected and to their family. Additionally, it is often important when deciding on possible clinical treatments. For example, to potentially preserve, maintain or restore fertility,” said Robin Lovell-Badge, head of the Crick’s Stem Cell Biology and Developmental Genetics Laboratory.
More information:
Nitzan Gonen et al, In vitro cellular reprogramming to model gonad development and its disorders, Science Advances (2023). DOI: 10.1126/sciadv.abn9793. www.science.org/doi/10.1126/sciadv.abn9793
Provided by
Bar-Ilan University
Citation:
Stem cell model allows researchers to explore the earliest stages of sex determination in mice and humans (2023, January 4)