Recent lab studies have shown that aging is a reversible process, an advancement that has prompted scientists to seek ways to stop the functional decline of cells and tissues, as well as restore their regenerative capacity.
This includes researchers at the University at Buffalo, where chemical engineer Stelios Andreadis showed that the embryonic gene NANOG could reprogram senescent (aged) adult stem cells and skeletal muscle cells, thereby reversing the hallmarks of aging.
How exactly NANOG works, though, has been a mystery.
Now, two new studies from Andreadis’ lab are helping to answer this question. One in Cell Reports explores the role NANOG plays in restoring mitochondrial function in aging stem cells. The other, published Feb. 16 in Nature Communications, sheds light on how it reverses aging in skeletal muscle.
The works builds upon the scientific community’s understanding of NANOG, which is named for the mythical land of youth in Irish folklore, and could help lead to the development of medicines that mimic the gene.
“With these studies, we discovered that NANOG reverses cellular senescence by restoring metabolic pathways that are active in younger cells. This brings us closer to developing improved treatments that will help alleviate suffering worldwide for people struggling with age-related illnesses,” said Andreadis, Ph.D., SUNY Distinguished Professor in the Department of Chemical and Biological Engineering at the UB School of Engineering and Applied Sciences.
Restoring mitochondrial function in aging stem cells
In Cell Reports, the research team focused on senescent mesenchymal stem cells. These are aging cells with greatly diminished ability to divide and grow.
Within these cells, the team found that glycolysis and mitochondrial respiration were compromised. The condition led the cells—in an effort to find a new energy source—to rewire their metabolism to breakdown an amino acid called glutamine. This action led to an accumulation of urea within the cells, which further hampered the mitochondria’s ability to provide energy to the cells and, thus, caused more aging.
To counter this metabolic rewiring, the team restrained an enzyme known as Glutaminase 1, which blocked the cells from breaking down glutamine.
“This partially restored mitochondrial function and decreased hallmarks of cellular senescence in animal models,” said the study’s lead author Debanik Choudhury, a Ph.D. candidate in Andreadis’ lab.
The team observed similar results in cells from patients with Hutchinson-Gilford progeria syndrome, a rare progressive genetic disorder that causes children to age rapidly.
Reversing aging in skeletal muscle
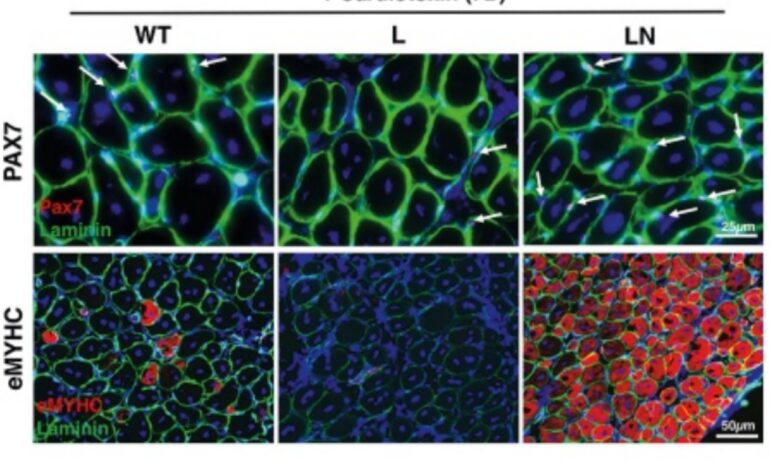
In Nature Communications, researchers investigated age-related metabolic changes that occur in aged and rejuvenated myoblasts, which are cells that make up muscle tissue.
These experiments, which used both in vitro and in vivo models of aging, revealed that myoblasts suffer from impaired glycolysis and insulin resistance. The experiments also showed that myoblasts generate adenosine triphosphate (an organic compound that provides energy for cellular processes) by breaking down methionine, an essential amino acid that’s also found in meat, fish, and dairy products.
This process produces significant levels of ammonium that may worsen cellular aging.
To fight this problem, the team expressed—the process by which the information encoded in a gene is turned into a function—NANOG. In turn, this suppressed the production of methionine adenosyltransferase 2A—the first enzyme in the methionine pathway—leading to decreased ammonium, restored insulin sensitivity, increased glucose uptake, and enhanced muscle regeneration post-injury.
Also, researchers found that blocking of methionine adenosyltransferase 2A activates signaling of Akt2—an enzyme involved in insulin signaling. It also repairs pyruvate kinase, restores glycolysis, and enhances regeneration, all of which leads to significant enhancement of muscle strength in a mouse model of premature aging.
Nika Rajabian, Ph.D., a former student in Andreadis’ lab, is the study’s lead author, and Kirkwood Personius, PT, Ph.D., associate clinical professor in the Department of Rehabilitation Science in the UB School of Public Health and Health Professions, collaborated on the work.
“Our investigation indicates that inhibiting methionine metabolism may restore age-associated impairments with significant gain in muscle strength and capacity for healing,” said Rajabian.
“Because the studies implicate metabolic pathways, this could lead to the development of small molecules—in other words, medicines—that mimic NANOG in restoring metabolism and reversing cellular hallmarks of aging such as inflammation and DNA damage,” Andreadis said.
More information:
Debanik Choudhury et al, Inhibition of glutaminolysis restores mitochondrial function in senescent stem cells, Cell Reports (2022). DOI: 10.1016/j.celrep.2022.111744
Nika Rajabian et al, Methionine adenosyltransferase2A inhibition restores metabolism to improve regenerative capacity and strength of aged skeletal muscle, Nature Communications (2023). DOI: 10.1038/s41467-023-36483-3
Provided by
University at Buffalo
Citation:
To reverse aging in stem cells, NANOG gene ‘rewires’ metabolic networks (2023, February 17)