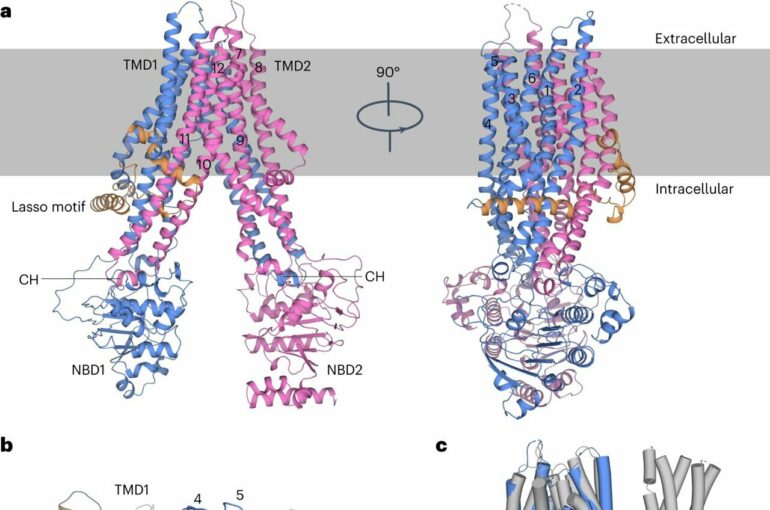
A research team led by Prof. Chen Yuxing and Prof. Zhou Cong from the University of Science and Technology of China (USTC) of the Chinese Academy of Sciences (CAS) recently utilized single-particle cryogenic electron microscopy (cryo-EM) to decipher the 3D structure and molecular mechanism of the platelet drug transporter ABCC4. The study was published online in Nature Cardiovascular Research.
In cases of vascular injury and bleeding, platelets need to be activated to promote blood clotting. Drugs such as aspirin and dipyridamole are commonly used in clinical practice to inhibit platelet function for the treatment of cardiovascular diseases.
ABCC4 is an important transporter protein belonging to the ABCC subfamily of ATP-binding cassette transporters. It is known to mediate the efflux of anticancer, antiviral, and antibacterial drugs. It is also involved in the transport of various physiological substrates. ABCC4 is highly expressed in platelets and is involved in the transport of platelet agonists and antagonists, making it a potential drug target for preventing cardiovascular diseases. However, the 3D structure of ABCC4 and the molecular mechanism of its broad-spectrum substrate transportation remain unknown.
Using cryo-electron microscopy, the researchers obtained the 3D structures of human ABCC4 in its apo form (without ligand), bound to three different substrates (the TXA2 analog U46619, aspirin, and dipyridamole), and simultaneously bound to U46619 and ATP. Based on structural analysis and biochemical experiments, they elucidated the molecular mechanism of ABCC4 in binding and transporting broad-spectrum substrates, including platelet agonists and antagonists.
The researchers found that the apo-form ABCC4 adopts an inward-facing conformation with the two nucleotide-binding domains (NBDs) separated from each other. The two transmembrane domains (TMDs) form a compatible substrate-binding pocket that extends toward the cytosol. This binding pocket is composed of a central hydrophobic cavity and peripheral polar amino acids.
The hydrophobic cavity stabilizes the hydrophobic core of the substrate, while the polar amino acids form hydrogen bonds with the polar groups of the substrate. The binding of ATP leads to the dimerization of the two NBDs, bringing the two TMDs closer together. As a result, ABCC4 undergoes a conformational transition from an inward-facing conformation to an outward-facing occluded conformation, closed to both the cytosol and extracellular sides.
Through structural analysis combined with biochemical experiments, the researchers demonstrated that TXA2 and aspirin are substrates of ABCC4. They also discovered that dipyridamole is a potent competitive inhibitor of ABCC4, thereby elucidating its molecular mechanism when used in combination with aspirin in clinical practice.
This study revealed the molecular mechanism by which ABCC4 recognizes broad-spectrum substrates, and provided a structural basis for the rational design of platelet antagonists targeting ABCC4 and the prevention of cardiovascular diseases.
A commentary article said that the structural elucidation of the ABCC4 transporter represents a crucial first step in understanding the key molecular mechanisms underlying the transport of platelet substrates into dense granules and efflux of drugs from platelets, which lays the foundation for comprehending platelet interactions and designing specific platelet antagonists.
More information:
Yu Chen et al, Structural insights into human ABCC4-mediated transport of platelet agonist and antagonist, Nature Cardiovascular Research (2023). DOI: 10.1038/s44161-023-00289-9
Anish V. Sharda et al, Insights into platelet pharmacology from a cryo-EM structure of the ABCC4 transporter, Nature Cardiovascular Research (2023). DOI: 10.1038/s44161-023-00293-z
Provided by
University of Science and Technology of China
Citation:
Unveiling the 3D structure and molecular mechanism of platelet drug transporter ABCC4 (2023, July 28)