by Instituto Nacional de Ciência e Tecnologia de Biologia Estrutural e Bioimagem (INBEB)
In a study published in Science Advances, researchers from the Federal University of Rio de Janeiro (UFRJ) and the German Center for Neurodegenerative Diseases (DZNE-Berlin) shed light on the intricate dance between the prion protein and copper ions in the physiopathology of live cells.
The research paves the way for potential treatments addressing copper-bound prion protein clusters to prevent abnormal solid formation and mitigate neurodegenerative outcomes.
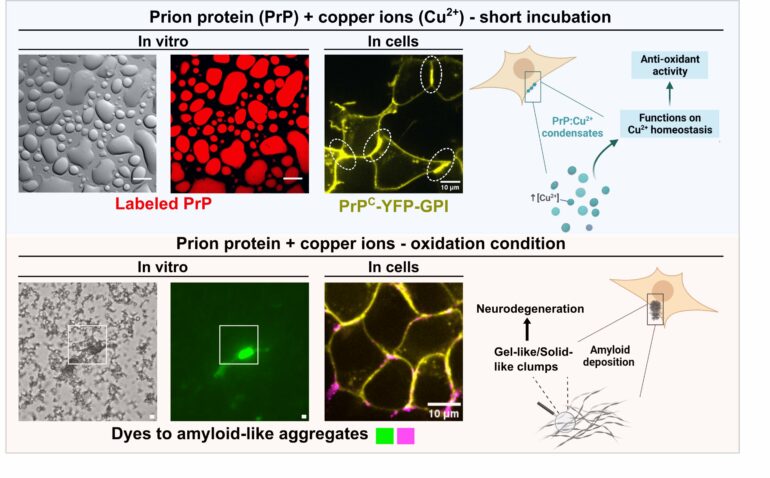
Like oil droplets in water, cells harbor membrane-bound organelles that play crucial roles in cellular function. The research contributes to the understanding of a new layer of complexity with the existence of membrane-less organelles or condensates formed through phase separation, which are protein-rich assemblies with unique liquid-like properties and dynamic functions. Notably, proteins linked to neurodegenerative diseases undergo phase separation, suggesting a potential link between liquid condensates and subsequent aggregation.
The prion protein (PrP), associated with fatal brain diseases like ‘mad cow’ disease, has long been known to interact with copper ions in brain cells. Led by Mariana Do Amaral, a graduate student under the supervision of Professor Yraima Cordeiro (UFRJ) and Professor Susanne Wegmann (DZNE-Berlin), the study demonstrates that PrP can form dynamic liquid condensates at the cell surface, potentially acting as scavengers for excessive copper ions.
Do Amaral, the paper’s first author, explains, “For over 20 years, research has hinted at copper binding to PrP and its role in abnormal folding. Our hypothesis was that PrP acts as a copper buffer via liquid–liquid phase separation, protecting cells from excess of copper.”
The findings highlight the biological significance of liquid–liquid phase separation in regulating copper homeostasis by PrP. The dynamic nature of PrP condensates, accumulating copper ions, suggests a finely tuned mechanism. Intriguingly, exposure to oxidative stress, a commonality in diseased or aged brains, led to a transition from liquid to solid, resembling clumps associated with neurodegeneration.
This study not only deepens our understanding of prion diseases but also opens avenues for potential interventions targeting copper-bound prion protein condensates to prevent abnormal solid formation and mitigate neurodegenerative outcomes.
The research utilized advanced biophysical techniques, including X-ray photon correlation spectroscopy (XPCS) at the new Brazilian synchrotron light source (Sirius-LNLS) and live cell fluorescence recovery after photobleaching at Charité-University Medicine Berlin.
More information:
Mariana Juliani do Amaral et al, Copper drives prion protein phase separation and modulates aggregation, Science Advances (2023). DOI: 10.1126/sciadv.adi7347
Provided by
Instituto Nacional de Ciência e Tecnologia de Biologia Estrutural e Bioimagem (INBEB)
Citation:
When physics meets biology: Prion protein orchestrates liquid–liquid phase separation with copper (2023, December 1)