Zinc is one of those micronutrients that many people know they need but are otherwise a little vague on the particulars.
Unlike, say, calcium, which most people know can be gained from a glass of milk, or the potassium found in a banana, sources of zinc sometimes aren’t as well-known.
The unknowns about zinc further extend to how it works in the body. While research has demonstrated that zinc is essential for host of vital functions—from cell growth and proliferation to DNA creation, immune system support, building proteins and many others—not much has been known about how zinc does its work. In fact, a lot of what scientists know about how zinc functions in the body, especially its role in growth, has been learned by studying its absence in cases of zinc deficiency.
However, newly published research led by Amy Palmer, a professor in the University of Colorado Boulder Department of Biochemistry, sheds new light—fluorescent light, in fact—on zinc’s role in cell growth. The findings are published in the journal Cell Reports.
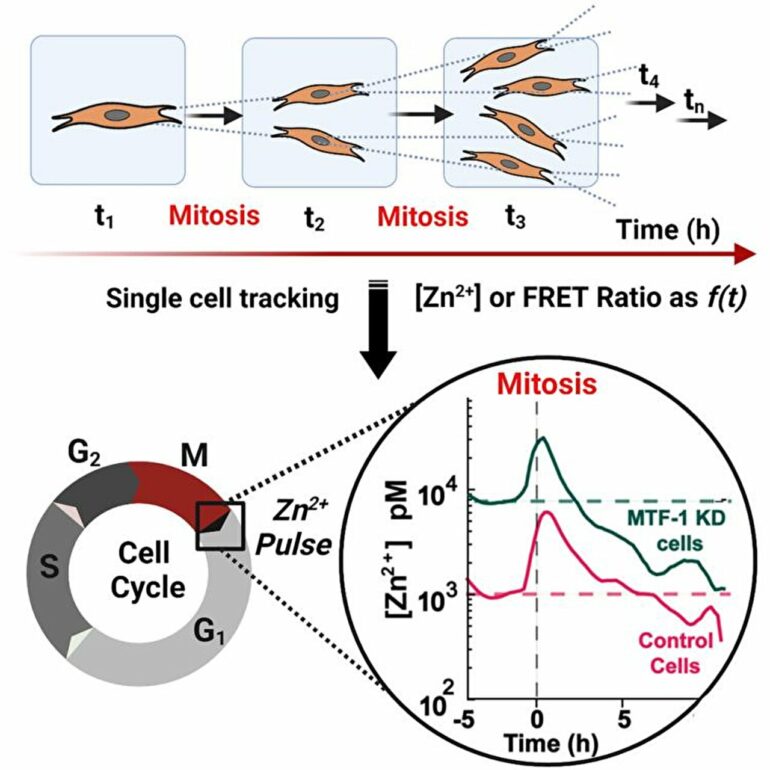
The research shows that when zinc levels are too low or too high, all cell proliferation stops until zinc levels come back into an acceptable range. It also revealed a phenomenon the researchers called a “zinc pulse”—right after a cell divides, it experiences a transient increase in zinc that comes back down after about an hour.
Palmer and her research colleagues, post-doctoral research associate Ananya Rakshit and graduate student Samuel Holtzen, were able to arrive at this new understanding of zinc’s vital role by using genetically encoded fluorescent sensors that change color and give off light when zinc binds to them.
“For the field, these fluorescent sensors were a big breakthrough because they allowed us to measure and quantify zinc in individual cells over many hours,” Palmer explains. “We can watch the zinc as the cell gets ready to divide, as it divides and as the two daughter cells go through the same process.
“We need to understand at the cellular level why is it that zinc is required, where is it required, how much is required. One missing piece of the puzzle, particularly when we think of zinc supplementation, is understanding and knowing when cells need zinc and how much they actually need.”
Using fluorescence
Palmer, who is internationally recognized for her work in developing the fluorescent sensors that detect metals in cells without disrupting cell function, and her research colleagues used a bit of biochemistry and a bit of engineering to create a sensor that will bind to zinc and only zinc.
“These fluorescent reporters are less perturbing to cells, letting them naturally cycle, and they’re really the wave of the future for this field of research,” Palmer says. “My colleague Sabrina Spencer really pioneered the approach of studying naturally cycling cells, and we learned a lot from her and her lab. Our angle was to take these fluorescent reporters and create some specifically for zinc.”
When Palmer initiated her lab at CU, she and her colleagues began developing these fluorescent sensors, building on post-doctoral research that Palmer completed with her advisor, Roger Tsien. Tsien won the Nobel Prize in Chemistry for discovering and developing the green fluorescent protein, which he and other scientists used to track when and where certain genes are expressed in cells.
“What’s really fun about these fluorescent sensors is they’re made out of proteins that are genetically encoded,” Palmer says. “They have a DNA sequence, and that one piece of DNA encodes a protein that will bind to zinc.
“This color switch when it binds to zinc specifically, this was a big breakthrough. It’s easy to get a very small response, but it’s harder to get a really big, robust response that can be used to track cells over 60 hours. We went through a lot of iterative optimization of our tools to get them to work the way we want.”
The effort paid off, though, because a lot of previous research added chemicals to cells to stop them from dividing or removed their growth serum—a process that could also remove zinc. Then, removing the chemical or adding the growth serum reinitiated cell division, aligning the cells so that they were all doing the same thing at the same time. That scenario, however, is not representative of what happens in a human body.
By introducing the fluorescent reporters to cells, Palmer and her colleagues could not only measure zinc levels, but also track each individual cell over 60 hours. Working with naturally cycling cells allowed the cells do their normal business in real time. Then, the researchers computationally figured out what state each cell was in and how much zinc it contained at each point during that time.
Implications for nutrition and disease
Palmer’s research was not only important because of the innovative tools being developed and used to study the cell cycle, but because zinc’s essentiality is not widely known yet the impacts of zinc deficiency can be significant. About 17% of the world’s population is zinc deficient and zinc deficiency represents a public health crisis in some parts of the world.
Severe zinc deficiency can result in slowing or cessation of growth and development, delayed sexual maturation, impaired immune function and wound healing and many others. However, scientists are just now beginning to understand when cells need zinc and how much of it they need.
By using fluorescent sensors to track zinc uptake in individual cells over 60 hours, Palmer and her co-researchers were able to discover the zinc pulse that occurs right after a cell divides.
“We don’t yet know exactly why that happens, but we speculate that the two new daughter cells need to bring in a lot of zinc to set up growth in the individual cell,” Palmer says. “If they don’t have that pulse then they can’t keep going and they have to pause.”
The researchers also saw that zinc levels need to be just right—if they’re too high or too low then cell function pauses until zinc levels return to normal. During that pause, they observed that cells struggled to make DNA.
Building on the results of the recently published study, undergraduate researchers in Palmer’s lab are studying the very high levels of zinc often found in breast cancer cells and why those cells don’t pause in response to high zinc levels the way healthy cells would. It’s almost as though cells have a safety switch that cancer is somehow able to bypass, Palmer says.
Digging deeper into when and why cells need zinc and how much of it may “have implications for understanding human nutrition at the whole-organism level, implications for understanding zinc dysregulation or dysfunction in disease,” Palmer says. “We’re really working to understand that set point and that fundamental mechanism that each cell has where it senses its zinc status and how, within a certain range, it can regulate how much zinc it has.”
More information:
Ananya Rakshit et al, Human cells experience a Zn2+ pulse in early G1, Cell Reports (2023). DOI: 10.1016/j.celrep.2023.112656
Provided by
University of Colorado at Boulder
Citation:
The right zinc levels are key to human health, researchers find (2023, July 26)