Scientists from within the Antibody and Vaccine Group at the University of Southampton have gained novel insights into how an important class of immune receptors called tumor necrosis factor receptors (TNFR) are activated.
The work, published in the journal Communications Biology, investigates a class of receptors present on immune cells called TNFR. These receptors, such as CD40, 4-1BB and OX40, are key in helping the immune system fight pathogens and cancer cells. Accordingly, antibody drugs which are designed to specifically target and activate these receptors (called agonists) have been developed for cancer treatment.
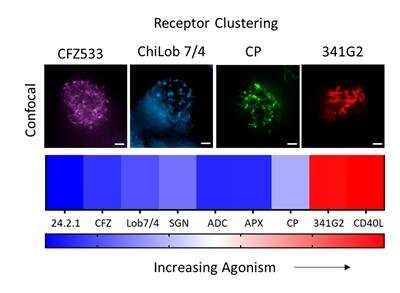
The mechanism by which these receptors are activated on the cell surface is important for designing optimal drug formats; however, to date it is not fully understood. Previous work showed that receptor clustering, redistribution of receptors dispersed over the cell surface into localized clusters, is essential for TNF receptor activation, and it is commonly believed that larger clusters induce more potent activation.
The current study, led by Dr. Ben Yu and Professor Mark Cragg at the Centre for Cancer Immunology, with colleagues across the University and at ONI UK, employed a set of unique reagents developed at Southampton targeting CD40, 4-1BB and OX40, as well as a new super-resolution microscopy acquired through funding from from the Mark Benevolent Fund, to address how differential receptor clustering mediates receptor activity.
Results from the study confirmed that TNF receptor activation absolutely requires receptor clustering but interestingly, disproved the commonly held belief that larger clusters induce more receptor activation. Rather, the study finds that agonists that induced smaller clusters—but with higher receptor density—mediated better TNF receptor activity than those which induced larger clusters.
In addition to receptor size, the study reveals that one of the most potent antibody agonists targeting CD40 induced a novel rod-shaped clustering structure, which could potentially explain the super-agonistic nature of that antibody. These findings add significant insight into how TNF receptors cluster to mediate immune activation and will help guide future development of therapeutic antibodies targeting TNF receptors.
Signal transduction without signal-receptor clusters can direct cell movement
More information:
Xiaojie Yu et al, TNF receptor agonists induce distinct receptor clusters to mediate differential agonistic activity, Communications Biology (2021). DOI: 10.1038/s42003-021-02309-5
Provided by
University of Southampton
Citation:
How density governs receptor activation on immune cells (2021, June 25)
retrieved 25 June 2021
from https://phys.org/news/2021-06-density-receptor-immune-cells.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.