Advances in transmission electron microscopy (TEM) can allow cryo-imaging of biological and biochemical systems in liquid form, however, such approaches do not possess advanced analytical capabilities. In a new report now published on Science Advances, A. A. El-Zoka and an international team of researchers in Germany, Canada, France, and the U.K., used atom probe tomography to analyze frozen liquids in three-dimensions (3-D) with sub-nanometer scale resolution. In this work, the team first introduced a specimen preparation strategy using nano-porous gold and used ice formed from high-purity deuterated water (hard water) alongside a solution of sodium chloride (50 mM) dissolved in high-purity deuterated water. They then analyzed the gold-ice interface to reveal increased solute concentrations across the interface. The scientists explored a range of experimental conditions to understand atom probe analyses of bulk aqueous specimens. Then they discussed the physical processes associated with the observed phenomena. The study showed the practicality of using frozen water as a carrier for near-atomic-scale analyses of objects in solution via atom probe tomography.
Transmission electron microscopy and atom probe tomography
Transmission electron microscopy (TEM) has undergone significant progress in recent decades, partly leading to the 2017 Nobel prize in Chemistry, due to the innovation of cryo-electron microscopy (cryo-EM) to determine the high-resolution structure of biomolecules in solution. The cryo-EM technique notably offers the ability to freeze specimens rapidly so that water molecules present in the specimens turn into transparent ice crystals. Tremendous parallel efforts have similarly established atomically resolved electron tomography methods to accomplish groundbreaking discoveries in materials science. Despite the powerful analytical capabilities, the approaches cannot readily measure the atomic-scale composition of a specimen. Here, El-Zoka et al. described the analysis of micron-thick layers of frozen water formed on nanoporous gold (NPG), with typical applications in catalysis, electrochemical sensing and actuation due to a high surface-area-to-volume ratio and gold-rich surface. The team therefore used NPG as a hydrophilic (water-loving) substrate on which to analyze ice using atom probe tomography.
Sample preparation
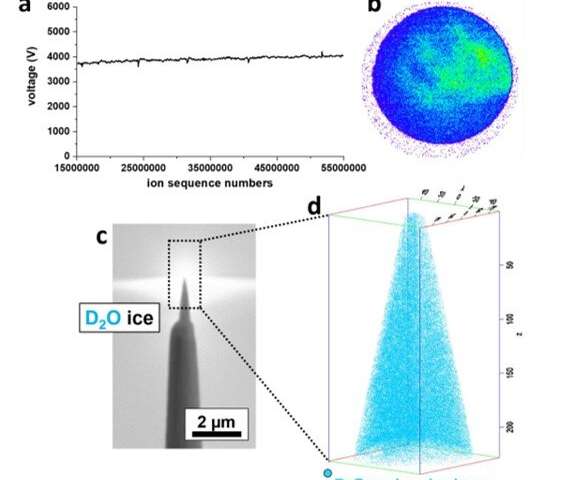
To prepare samples suited for field evaporation in an atom probe microscope, El-Zoka et al. used a blotting and plunge-freezing approach similar to that implemented in cryo-EM. For this, they chose an in-situ plasma focused ion beam approach (PFIB) at cryo-temperature. The arrangement allowed the preparation of a stable specimen composed of frozen liquid. They detailed a wide range of pulsed-laser atom probe data from pure deuterated water (D2O) and a D2O-based solution of sodium chloride. The team imaged and characterized small metallic objects floating in solution by analyzing data at the ice-NPG (nanoporous gold) interfaces. They discussed the physics of field evaporation to detect sets of molecular ions and their influence on the performance of cryo-atom probe tomography. The work provides a necessary step to investigate a new playing field for near-atomic-scale analysis of solute effects in confined freezing nano-objects and molecular or biological materials in their native environments.
Atom probe tomography of ice
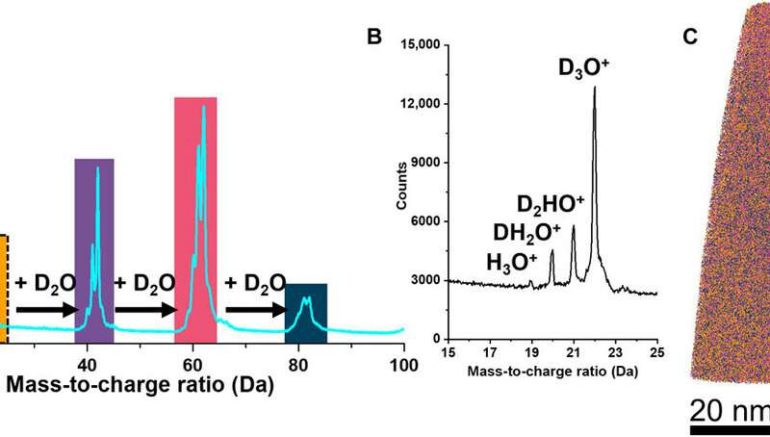
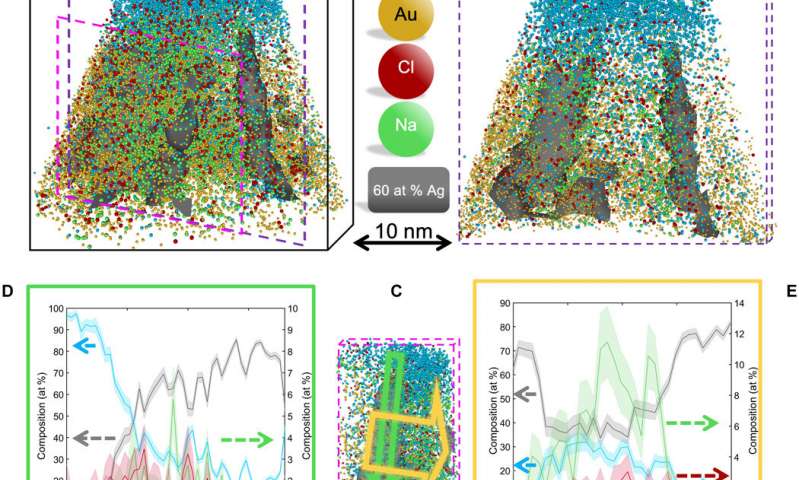
El-Zoka et al. combined atom probe tomography specimen preparation protocols to transfer environmentally sensitive specimens and repeatedly collected data displaying ice chemistry at near-atomic scale resolution. The device contained a laser-pulsing mode with a pulse of 20 to 100 petajoules and a pulse rate of 25 to 200 kHz. The team set the target evaporation rate in the setup to 0.003 or 0.005 ions per pulse by adjusting an applied direct current (dc) voltage (ranging between 2 to 5 kV) in the experiment. They obtained a summarized dataset indicating the smooth evolution of the applied direct current voltage during the experiment. The scientists notably detected cations from water evaporation in the form of singly charged molecular ions of one to five D2O molecules and detected such water clusters to be interchangeably protonated with H (hydrogen) and D (deuterium) atoms. Nevertheless, fully deuterated clusters dominated the mixture in abundance. In this way, the preliminary work showed the possibility of analyzing frozen liquid-metal interfaces.
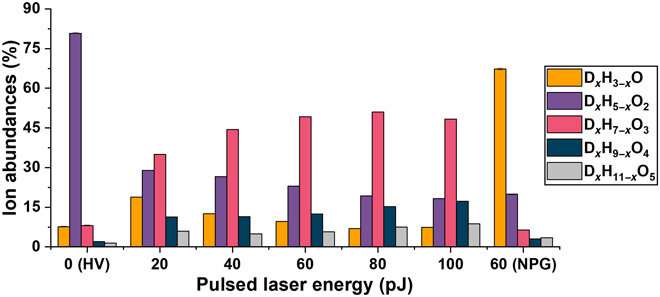
Background noise
The team also quantified the level of background to understand the sensitivity of the atom probe tomography-based analyses of solutions. The detected background levels were relatively high compared to usual analyses; however, this could be lowered by changing the experimental parameters. Since ice is a significantly poor heat conductor, the team lowered the repetition rate of the laser in the study to prevent a possible pileup of thermal pulses. The team showed how varying the pulse energy and pulse frequency allowed increased homogeneity of the field evaporation process with decreasing pulsing energies. Most of the observed background developed due to the field evaporation of water by the electrostatic field. A drop in the level of background could therefore be achieved by lowering the average temperature of the specimen, by lowering the average temperature of the specimen, or by lowering the average electrostatic field in the device. When using water as a carrier medium to analyze nanomaterials the experimental conditions require fine-tuning to maximize the signal-to-background ratio.
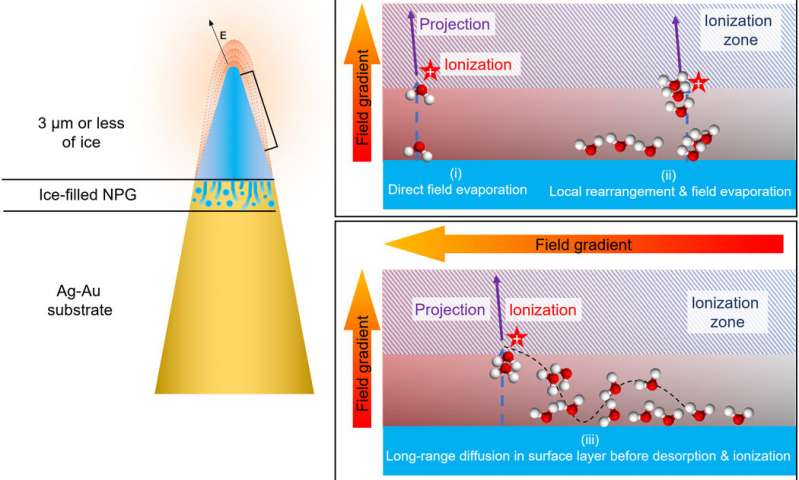
Outlook for chemical, biological and biochemical imaging.
In this way, A. A. El-Zoka and colleagues overcame the barriers of conventional focussed ion beam/atom probe tomography (FIB/APT) to analyze liquid layers and nanostructures encapsulated in liquid layers. The team used nanoporous gold (NPG) as a substrate to develop ice needles in combination with a cryo-plasma focused ion beam (cryo-PFIB) suited for atom probe analysis. The results showed the capability of analyzing bulk ice layers and probing encapsulated nano-ligaments alongside the surrounding solvated ions at near-atomic scale. The approach will pave the way to use nanoporous metals to routinely investigate liquid layers at encapsulated nanostructures. The chemistry of the metal and pore size can be optimized to improve observed aberrations at the ice-solid interface and within nanopores of materials. The set of experiments completed here allow a first and major step forward to develop near-atomic-scale analytical imaging of chemical, biochemical and biological systems.
Non-uniform evaporation prevents scientists from seeing every atom on a surface
More information:
El-Zoka, A. A. et al. Enabling near-atomic–scale analysis of frozen water, Science Advances, DOI: 10.1126/sciadv.abd6324
Chen C. C. et al. Three-dimensional imaging of dislocations in a nanoparticle at atomic resolution. Nature, doi.org/10.1038/nature12009
Hydrogen production from formic acid decomposition at room temperature using a Ag-Pd core-shell nanocatalyst. Nature Nanotechnology, doi.org/10.1038/nnano.2011.42
2020 Science X Network
Citation:
Near-atomic-scale analysis of frozen water (2020, December 11)
retrieved 14 December 2020
from https://phys.org/news/2020-12-near-atomic-scale-analysis-frozen.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.