Bringing a new drug to market costs billions of dollars and can take over a decade. These high monetary and time investments are both strong contributors to today’s skyrocketing health care costs and significant obstacles to delivering new therapies to patients. One big reason behind these barriers is the lab models researchers use to develop drugs in the first place.
Preclinical trials, or studies that test a drug’s efficacy and toxicity before it enters clinical trials in people, are mainly conducted on cell cultures and animals. Both are limited by their poor ability to mimic the conditions of the human body. Cell cultures in a petri dish are unable to replicate every aspect of tissue function, such as how cells interact in the body or the dynamics of living organs. And animals are not humans – even small genetic differences between species can be amplified to major physiological differences.
Fewer than 8% of successful animal studies for cancer therapies make it to human clinical trials. Because animal models often fail to predict drug effects in human clinical trials, these late-stage failures can significantly drive up both costs and patient health risks.
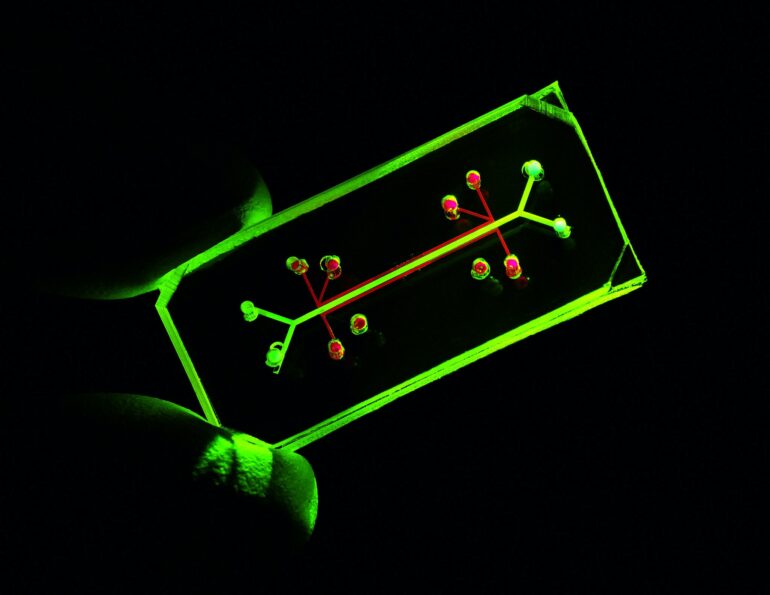
To address this translation problem, researchers have been developing a promising model that can more closely mimic the human body – organ-on-a-chip.
As an analytical chemist, I have been working to develop organ and tissue models that avoid the simplicity of common cell cultures and the discrepancies of animal models. I believe that, with further development, organs-on-chips can help researchers study diseases and test drugs in conditions that are closer to real life.
Organs-on-chips offer an alternative model for early-phase biomedical research.
What are organs-on-chips?
In the late 1990s, researchers figured out a way to layer elastic polymers to control and examine fluids at a microscopic level. This launched the field of microfluidics, which for the biomedical sciences involves the use of devices that can mimic the dynamic flow of fluids in the body, such as blood.
Advances in microfluidics have provided researchers a platform to culture cells that function more closely to how they would in the human body, specifically with organs-on-chips. The “chip” refers to the microfluidic device that encases the cells. They’re commonly made using the same technology as computer chips.
Not only do organs-on-chips mimic blood flow in the body, these platforms have microchambers that allow researchers to integrate multiple types of cells to mimic the diverse range of cell types normally present in an organ. The fluid flow connects these multiple cell types, allowing researchers to study how they interact with each other.
Microfluidics can be used for many applications in biological research.
This technology can overcome the…