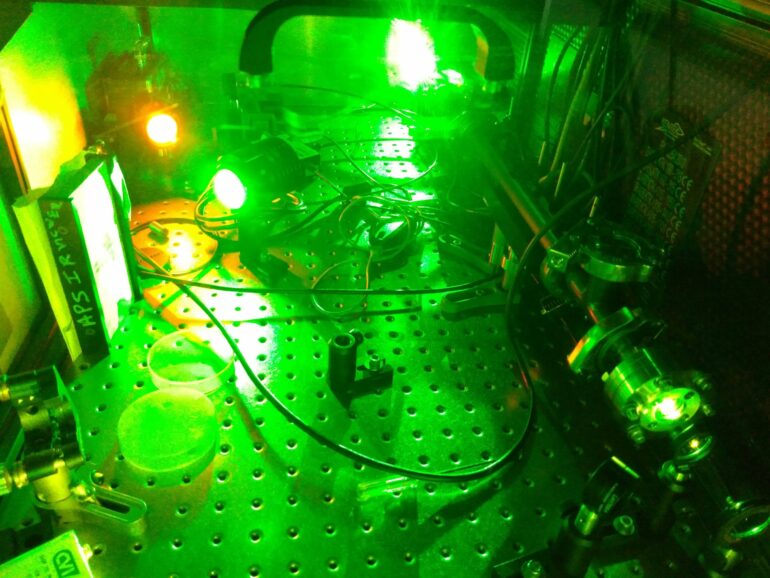
Lasers are intense beams of colored light. Depending on their color and other properties, they can scan your groceries, cut through metal, eradicate tumors, and even trigger nuclear fusion. But not every laser color is available with the right properties for a specific job.
To fix that, scientists have found a variety of ways to convert one color of laser light into another. In a study just published in the journal Physical Review Applied, scientists at the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory demonstrate a new color-shifting strategy that is simple, efficient, and highly customizable.
The new method relies on interactions between the laser and vibrational energy in the chemical bonds of materials called “ionic liquids.” These liquids are made only of positively and negatively charged ions, like ordinary table salt, but they flow like viscous fluids at room temperature. Simply shining a laser through a tube filled with a particular ionic liquid can downshift the laser’s energy and change its color while retaining other important properties of the laser beam.
“By adding a certain ion that has a particular vibrational frequency, we can design a liquid that shifts the laser light by that vibrational frequency,” said Brookhaven Lab chemist James Wishart, an expert on ionic liquids and a co-author on the paper. “And if we want a different color, then we can switch out one ion and put in another that has a different vibrational frequency. The component ions can be mixed and matched to shift laser colors by different degrees as needed.”
The paper describes using the method to achieve color changes that have been difficult to produce using other methods, including a shift from green laser light to orange—long-sought for medical applications such as treating skin and eye conditions.
Giving lasers good vibes
The idea grew out of a project to boost the capabilities of a unique, high-power carbon-dioxide (CO2) laser at Brookhaven Lab’s Accelerator Test Facility (ATF). Scientists use the ATF, a DOE Office of Science user facility, to explore innovative concepts ranging from laser-energized particle accelerators to compact and bright X-ray sources.
“ATF’s CO2 laser is the only ultra-short-pulse, long-wavelength laser in the world; there are experiments you can do there that you can’t do anywhere else,” said study co-author Rotem Kupfer, a former postdoctoral fellow at ATF. “Replacing the method of pumping this laser from commonly used electric discharge to optical excitation should improve the beam quality and the repetition rate to allow even better experiments.”
To create a laser with the appropriate wavelength (a.k.a. color) for optical pumping, the scientists sought to shift the wavelength of an existing laser. They chose the general approach of stimulated Raman scattering, which harnesses the vibrational frequencies of molecules in a solid, liquid, or gas.
“Basically, the laser deposits energy into the molecular vibrations—the squishing and stretching of the chemical bonds that make up the material. Then the photons (particles of light) that come out have the original energy minus the energy of those vibrations,” Kupfer said. The lower-energy photons have a longer wavelength, or in other words, a different color.
In gases, the process is fairly simple because you are dealing with single molecules. But those molecules have limited vibrational frequencies, which limits the types of shifts. And diffuse gaseous molecules mean scattering efficiency is low. Solids, with more tightly packed molecules, could improve efficiency. But their more complex vibrational frequencies complicate the recipe for growing such materials with the desired properties, so making these materials is costly.
“Liquids are somewhere in between,” Wishart said. “You’re still dealing with single molecules, but denser, meaning higher efficiency than gases. And with ionic liquids, you can engineer the molecules to give you the frequency you need.”
Optically transparent ionic liquids also make it easy to avoid background absorption of light, and their higher viscosity avoids laser scattering from acoustic waves, which competes with and diminishes the color-shifting effect in low-viscosity liquids like water.
As the scientists worked on choosing an ideal ionic liquid for pumping the CO2 laser, they realized the color-shift approach using ionic liquids had even broader appeal. In the paper, they describe its use in additional color changes, including the elusive green-to-orange shift.
“There are a lot of hard ways to do Raman shifting. But for this one, we just filled a tube with a properly selected ionic liquid, shot a laser in from one end and we got the color we wanted out—without any fine tuning,” Wishart said.
“Other methods for achieving such a color shift require complex optical setups or the use of toxic materials such as dyes dissolved in solvents,” Kupfer said. “Plus, those other processes ‘break’ the molecules; they wear out and have to be replaced. In our case, it is a balance sheet. The molecules stay unharmed.”
Wishart agreed: “It shakes up the molecules but doesn’t break them.”
The scientists say there is a range of improvements that could optimize the process, but that overall, made-to-order ionic liquids are a platform for efficient, simple, and adjustment-free laser color shifting for numerous industrial and technological purposes.
More information:
Rotem Kupfer et al, Raman Wavelength Conversion in Ionic Liquids, Physical Review Applied (2023). DOI: 10.1103/PhysRevApplied.19.014052
Provided by
Brookhaven National Laboratory
Citation:
New strategy uses ionic liquids to change laser colors with ease (2023, January 19)