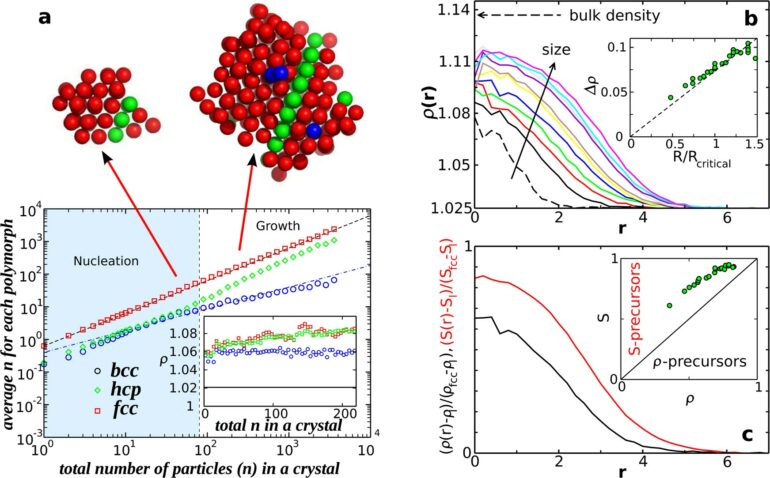
Crystallization in liquids is a fundamental phase transition. While for many years, the understanding of crystallization was governed by classical nucleation theory, recent research has shifted focus to non-classical pathways in crystallization. In a new study, scientists from the Institute of Industrial Science, The University of Tokyo, reveal that the crystal precursor structure, which forms spontaneously as a structural fluctuation in a supercooled liquid, has a critical impact on crystal nucleation and growth.
Crystallization, the formation of homogenous, ordered solids from liquids, is a crucial process in a variety of fields, ranging from atmospheric science to pharmaceuticals to semiconductor manufacturing. As such, an understanding of crystallization on a molecular level is a critical research area with wide applicability. For decades, crystallization has been understood in terms of classical nucleation theory (CNT). CNT states that microscopic solids (nuclei) form randomly and spontaneously from the liquid and begin growing into a crystal once they exceed a certain size. However, more recent research in the field has demonstrated that CNT is not always valid and that non-classical pathways need to be explored to fully understand the phenomenon of crystallization.
Studies on the structural properties of glass-forming liquids (liquids which form a non-crystalline, amorphous “solid” upon supercooling) have shown that, contrary to predictions by CNT, nucleation is not random. Instead, crystal nuclei are induced in specific preordered regions of the supercooled liquid that have local orientational symmetry which is consistent with the crystal. Moreover, recent research into fast crystal growth, which cannot be predicted by CNT, has cast doubt on one of the fundamental assumptions of CNT—that crystal growth rate is independent of interfacial tension (the propensity of a liquid to have a minimum free surface when in contact with another immiscible liquid).
To address these questions about CNT, a research team from the Institute of Industrial Science, The University of Tokyo (UTokyo-IIS), delved into the role of preordering on crystal growth and nucleation. The research team consisted of Professor Emeritus Hajime Tanaka of the Research Center for Advanced Science and Technology, UTokyo (formerly from Utokyo-IIS) and Dr. Yuan-Chao Hu, Yale University (formerly from Utokyo-IIS). The study, published in Nature Communications, highlights critical shortcomings in CNT and proposes critical modifications to address them.
In this study, the research team performed extensive molecular dynamics (MD) simulations of a supercooled nickel-aluminum alloy (NiAl). “We found that NiAl follows a non-classical crystallization pathway and that structural fluctuations in the precursors of crystals dramatically influenced crystal growth,” reveals Dr. Hu.
The research team then developed a novel “order-killing strategy” to suppress preordering. They found that the order-killing strategy successfully reduced crystallization rate over several orders of magnitude. “Preordering reduces interfacial energy,” explains Prof. Tanaka. “Our findings indicate that preordering and its associated reduction in interfacial energy are critical to crystal nucleation and growth, which exposes an important gap in CNT.”
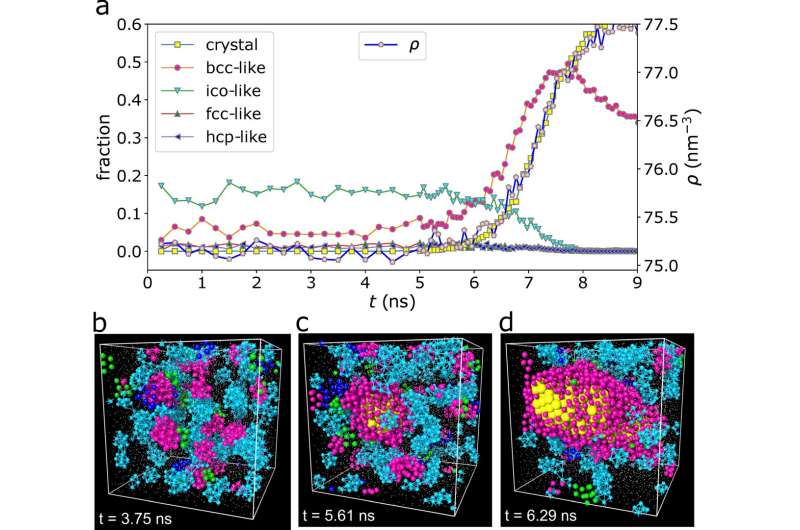
The figure depicts (a) the time-dependent fraction of crystallized atoms and their various structural orderings, indicating that crystal-like (here, bcc-like) preordering is a significant process in the growth of crystals. (b–d) show the different atomic configurations of the crystal at different times (t), indicating that preordering is transient and fluctuates in space, and that crystal nuclei are born from and grow from the crystal-like preordered regions. (c) highlights the critical nucleus in this condition. © Hajime Tanaka from University of Tokyo
Prof. Tanaka and Dr. Hu then accounted for interfacial energy in their simulations by including an interfacial energy-related factor. They then evaluated the interfacial energy-related factor in eight different systems with different bonding types and crystal structures. “Our findings suggest that liquid preordering could be the most important contributor to crystallization kinetics and glass formation. This could have a significant ripple effect in both fundamental science and industrial applications,” concludes Prof. Tanaka.
The findings of the study provide novel insights into crystallization kinetics. The implications of this study are sure to influence a wide-range of crystal-related applications, such as the control of silicon crystallization in the semi-conductor industry.
More information:
Yuan-Chao Hu et al, Revealing the role of liquid preordering in crystallisation of supercooled liquids, Nature Communications (2022). DOI: 10.1038/s41467-022-32241-z
Provided by
University of Tokyo
Citation:
Order up: New study reveals importance of liquid structural ordering in crystallization (2022, August 8)