Understanding the interactions between materials and chemical species is critical for engineers as it helps them determine their best uses for both day-to-day life and global-level applications.
Metal oxides, a binary material of metal and oxygen, greatly interest researchers because of their importance in transforming energy storage, production, and conversion. To further those possibilities, a team from the University of Pittsburgh Swanson School of Engineering has determined a new way to view how hydrogen and metal oxides interact.
“Numerous experimental techniques have been used to understand this phenomenon—from spectroscopy to catalysis” explained Giannis Mpourmpakis, associate professor and Bicentennial Alumni Faculty Fellow at the Swanson School. “These methods do provide a multifaceted view of how materials engage with chemical species, but they can be costly and time consuming.”
Mpourmpakis and his team rely upon their method of computational modeling and machine learning to effectively replace the traditional trial-and-error method of scientific research. Their virtual reactivity model accurately predicts what electrochemical conditions result when hydrogen is inserted into different metal oxides.
“The larger implications are that models like the one developed in this work—which takes into consideration local and global geometry of a material—can find diverse applications from energy storage to catalysis and the production of chemicals,” Mpourmpakis said. “Any application that is sensitive to the structure of a material can use this model to develop structure-property relationships and identify materials with desired performance.
“What’s more, computational modeling allows us to test and validate a large number of materials safely and effectively at a much lower cost with great success that other researchers can then further pursue.”
Pitt’s Swanson School has previously published research on how materials interact with their chemical environment in the past. In May 2022, research led by the same team began the creation of new, sustainable catalysts based on tungsten oxide and similar compounds. The project used computational simulations to understand how tungsten oxide interacts with hydrogen at the molecular level and the findings were verified through lab experimentation.
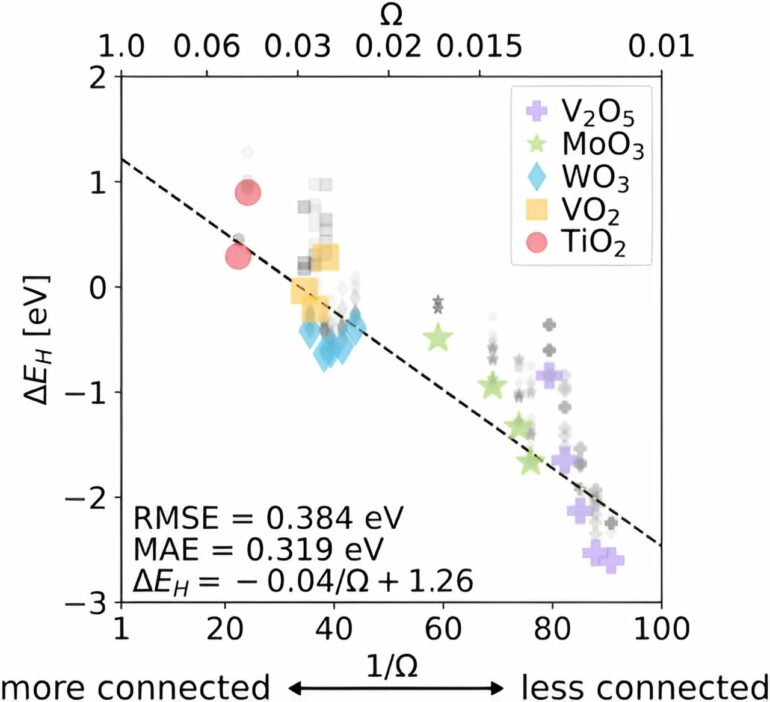
The paper, “Global and local connectivities describe hydrogen and intercalation in metal oxides,” was recently published in Physical Review Letters.
More information:
Evan V. Miu et al, Global and Local Connectivities Describe Hydrogen Intercalation in Metal Oxides, Physical Review Letters (2023). DOI: 10.1103/PhysRevLett.131.108001
Provided by
University of Pittsburgh
Citation:
Paper proposes a new way to understand how materials interact with hydrogen (2023, September 11)