A Freie Universität Berlin research team headed by quantum physicist Professor Christiane Koch has demonstrated how hydrogen molecules behave when they collide with noble gas atoms such as helium or neon. In an article published in the journal Science, the researchers describe how they used simulations to draw connections between data from experiments and theoretical models of quantum physics.
The study includes theoretical calculations as well as data collected in experiments with atoms and molecules conducted at TU Dortmund University and the Weizmann Institute of Science in Israel. The team was able to show that collisions change the way the molecules vibrate and rotate under the laws of quantum mechanics. Research in the field of quantum mechanics continues to gain importance in today’s world. Findings such as these can be applied in the development of mobile phones, televisions, satellites, and in medical diagnostic technology.
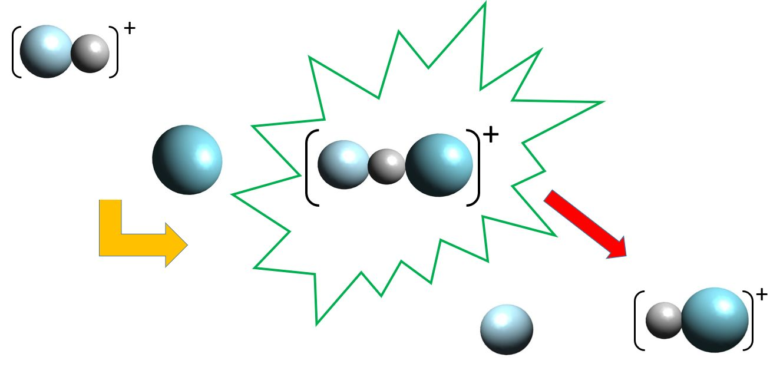
The quantum effect observed here is known as a Feshbach resonance. “For a brief moment after colliding, the hydrogen molecule and noble gas atom form a chemical bond and then separate again,” explains Professor Koch of Freie Universität Berlin.
However, despite the extremely detailed measurements and calculations for a comparatively small and simple system, researchers are still a long way from being able to reconstruct the full quantum mechanical characteristics of the hydrogen-noble gas collision. “This is due to one of the fundamental phenomena of quantum mechanics: when it comes to measurements, you can’t get around the basic principles of classical physics. That creates a dilemma: We are able to describe certain phenomena of quantum mechanics mathematically in abstract terms, but still need to use concepts from classical physics to fully understand them,” Koch explains.
Quantum effects—meaning types of behavior that cannot be explained with the rules of classical physics—appear when atoms and molecules can no longer be sufficiently described by the place they occupy and the speed at which they move. “They display characteristics that we associate with wave dispersion, such as interference, meaning the constructive or destructive layering of waves,” says Koch. On top of that, there are other phenomena such as entanglement, which happens when quantum mechanical objects exert immediate influence on one another despite being spatially distant.
Quantum effects typically appear in the realm of very small objects such as atoms and molecules, and when these objects are under little influence from their environment. The latter is achieved for very short bursts of time or under extremely low temperatures near absolute zero (-273.15°C). “Under these circumstances, only a small amount of so-called quantum states are available to these particles. The system basically behaves in an orderly way,” says Koch.
Higher temperatures allow for greater numbers of quantum states in the particles, and quantum mechanical effects tend to even out when distributed as a statistical average across various states, and thus essentially disappear from view. In this state, the system behaves more randomly and can be described using statistics. So far, even the coldest atom-molecule collisions have displayed this statistically predictable behavior. “That has made it nearly impossible to come to any conclusions as to the interaction between the atoms and molecules, meaning we couldn’t make a direct connection between real-life experimental data and theoretical models,” Koch explains.
More information:
Baruch Margulis et al, Tomography of Feshbach resonance states, Science (2023). DOI: 10.1126/science.adf9888
Provided by
Freie Universität Berlin
Citation:
Quantum effects detected in hydrogen and noble gas collisions (2023, June 5)