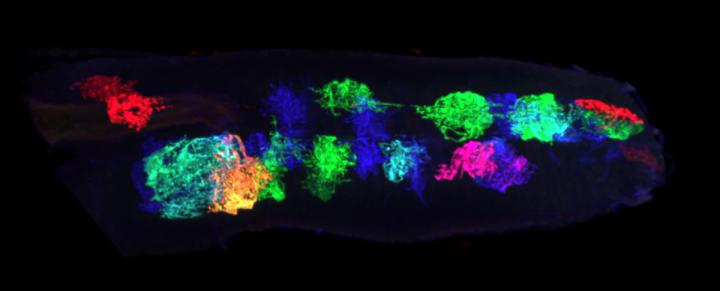
Researchers exploring the developing central nervous system of fruit flies have identified nonelectrical cells that transition the brain from highly plastic into a less moldable, mature state.
The cells, known as astrocytes for their star-like shapes, and associated genes eventually could become therapeutic targets, said University of Oregon postdoctoral researcher Sarah Ackerman, who led the research.
“All of the cell types and signaling pathways I looked at are present in humans,” Ackerman said. “Two of the genes that I identified are susceptibility genes linked to neurodevelopmental disorders including autism and schizophrenia.”
The failure to close so-called critical periods of brain plasticity in development, when learning occurs rapidly and helps mold the brain, she added, also is associated with epilepsy.
The discovery is detailed in a paper published online April 7 in the journal Nature. The research was done in the Institute of Neuroscience lab of co-author Chris Doe, a Howard Hughes Medical Institute investigator and professor in the UO Department of Biology.
Astrocytes are glial cells found in large numbers in the central nervous system. They play diverse roles depending on what regions in the brain and spinal cord where they are active. They are, Ackerman said, “the guardians of synapses in terms of assuring proper functioning in both their formation and later performance.”
In the research, Ackerman focused on the motor circuitry of Drosophila melanogaster larvae over specific points in development. These invertebrate fruit flies are standard research models that are easily open to rapid genetic exploration of molecular mechanisms.
Ackerman used optogenetics, a light-based technology, to selectively turn motor neurons off and on. She found that these neurons exhibit striking changes to their shape and connections—the plasticity—in response to the manipulations.
Curiously, Ackerman and colleagues saw astrocytes pouring into the nervous system, extending fine projections and enveloping neuronal connections at the right time to switch the circuitry from plastic to stable states.
Ackerman then screened for candidate genes associated with astrocytes to determine which molecular pathways direct the window to close and shut down motor plasticity.
That work pointed directly at neuroligin, a protein on astrocyte projections, that binds to neurexin, a receptor protein on dendrites from developing neurons. Eliminating that genetic pathway extended plasticity, while precocious expression of these proteins closed plasticity too early in development.
Such changes in the timing of plasticity were also found to later impact behavior. Extending plasticity resulted in abnormal crawling of the larvae. Extending critical periods of plasticity in human development, Ackerman said, has been linked to neurodevelopmental disorders.
A tragic human example of how this critical period is vital, Doe said, may be the case of abandoned Romanian children found in an orphanage in the 1980s. Hundreds of babies had been neglected except when they were fed or washed, according to news reports.
The neglect would have occurred during that key period of plasticity when experiences and learning mold the brain, Doe said. When later removed from the orphanage four of every five of the children were unable to engage socially, according to research that followed the children into adulthood.
“My work was designed to understand what causes the shift from having a really malleable and flexible child brain to one that is more fixed and stable,” Ackerman said. “Rather than focusing on the neurons, I found that these really cool star-shaped cells called astrocytes are coming into the nervous system and telling the neurons to shift from being really malleable into a stable state.”
The implications of Ackerman’s research are potentially profound, Doe said.
“If we can understand that mechanism of the closing of this critical developmental period, we could possibly reopen plasticity in older people who want to, say, learn a new language or learn a new task,” Doe said.
That therapeutic potential is a long way off, the UO researchers said, but it is a major future goal. Ackerman’s research will next move into similar studies in vertebrates, specifically using zebrafish, which were developed into a model organism for medical research at the UO in the 1970s.
Any move into therapeutics, Ackerman cautioned, will require precise titration of any drugs that may be developed so that they find “the sweet spot for plasticity.”
Acute breakdown of the glial network in epilepsy
More information:
Sarah D. Ackerman et al, Astrocytes close a motor circuit critical period, Nature (2021). DOI: 10.1038/s41586-021-03441-2
Provided by
University of Oregon
Citation:
Researchers identify pathway that transitions brain from plasticity to stability (2021, April 8)
retrieved 8 April 2021
from https://medicalxpress.com/news/2021-04-pathway-transitions-brain-plasticity-stability.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.