The universe is more than 13 billion years old and space is often depicted as a vast, empty vacuum. Other than planets and stars, there’s nothing there, right? Actually, space is littered with complex, carbon-based molecules. However, the range of molecules and the chemistry involved in their formation remains largely mysterious.
There have been tantalizing hints of complex astrochemistry. For example, prebiotic molecules like amino acids and nucleobases have been detected in meteorites—the most famous landed in 1969 near Murchison, around 140 km north of Melbourne.
But to understand the molecular makeup of space, astronomers and astrochemists must go beyond analyzing the meteorites that happen to crash into Earth.
To do this, astronomers measure stellar radiation using telescopes, while other scientists simulate interstellar conditions in the laboratory—more on how our team uses this technique later.
Observing astronomical molecules
Of the approximately 240 molecules now discovered in space, most have been revealed using radio telescopes.
The James Web Space Telescope (JWST)—the largest space telescope ever launched—is designed to image very distant objects and the emission from chemical species in the mid-infrared, which can be used to identify elements and molecules.
The high sensitivity and resolution of the JWST has already made possible several recent milestone observations of astronomical molecules that help unravel the nature and origin of chemical complexity in the universe.
These include recent evidence of complex organic molecules in the galaxy SPT0418-47, located 12.3 billion light-years away—the most distant and oldest organic molecules that have ever been detected.
The molecules are polyaromatic hydrocarbons (PAHs) consisting of two or more fused aromatic rings of carbon, which are ubiquitous on earth, found in living systems, fossil fuels, and are implicated in the chemistry involved in the origin of life.
Another key discovery is the observation of methyl cation methylium (CH3+) in the disk surrounding a newly formed star.
That region is exposed to intense ultraviolet light from the hot young star, providing the energy necessary to form CH3+, which has the molecular structure equivalent to methane minus one hydrogen atom and plays an important role in the formation of more complex carbon-based molecules.
These and other ingredients for complex chemistry are ejected by novae, supernovae and other massive cosmic events into the vast region between stars—the interstellar medium.
Although we can’t just travel lightyears away to collect and study interstellar molecules, astronomers can detect molecules in the interstellar medium by collecting the light emitted by stars.
Since the light travels such an enormous distance before reaching the detectors on earth, it has an appreciable chance of being absorbed by molecules in interstellar clouds.
The wavelengths of light that are absorbed give a spectrum that contains fingerprints of the molecules in space.
Intriguingly, more than 500 absorption lines have been found in the visible and near-infrared region of the spectrum, which are known as the diffuse interstellar bands (DIBs). Several of the most prominent DIBs were first observed by Ph.D. student Mary Lea Heger in 1919 at the Lick Observatory in California (U.S.), with many more DIBs discovered since.
What are interstellar molecules made of?
While it is now clear that DIBs arise from molecules in the interstellar medium, the structure and composition of the molecules responsible for almost all DIBs remain a mystery.
In fact, only one molecule has been identified as the source of any features in the DIBs spectra, the carbon cluster buckminsterfullerene (C60+), which has the appearance of a tiny soccer ball.
Because C₆60+ is the only confirmed DIB carrier, it is reasonable to expect that other DIBs may be due to large carbon clusters consisting of between 10 and 100 atoms.
Carbon clusters have a diverse range of sizes and molecular shapes; however, the wide range of possible structures complicates their detection.
Simulating space
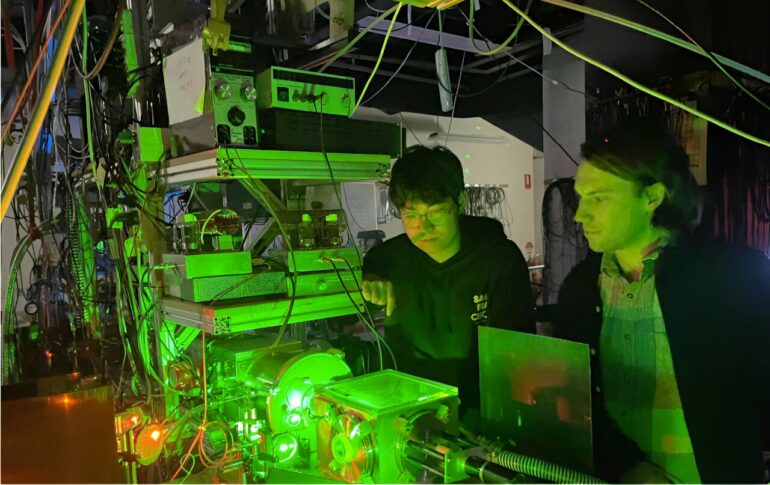
In the basement of the Chemistry building in the Laser Spectroscopy Laboratory, Dr. Samuel Marlton, Ph.D. candidate Chang Liu, and Professor Evan Bieske employ a home-built apparatus to generate, separate and isolate individual carbon cluster structures under gas-phase conditions that resemble the cold vacuum of space.
With this apparatus, the team compare the astrophysical data measured by astronomers with laboratory data for specific carbon cluster structures.
In recent work published in the Journal of Physical Chemistry A, the research team examined the absorption spectra of Colossal Carbon Rings, carbon aggregates containing between 14 and 36 atoms arranged as planar rings.
In that work, they found evidence for a carbon ring, C14+ (made from 14 carbons) that might contribute to the DIBs spectra.
It is an exciting result because almost all of the more than 500 DIBs are due to molecules that remain unidentified, which illustrates the ongoing mysteries surrounding the molecular makeup of interstellar space.
Although JWST is taking astronomical measurements at lower energy than reported here, both are part of the project to detect interstellar molecules through combined astronomical and laboratory investigations.
Together, these techniques are just beginning to reveal what is out there in the universe, and it’s so much more than empty space.
More information:
Samuel J.P. Marlton et al, Probing Colossal Carbon Rings, The Journal of Physical Chemistry A (2023). DOI: 10.1021/acs.jpca.2c07068
Provided by
University of Melbourne
Citation:
Simulating space to explore the great mystery of interstellar chemistry (2023, October 24)