A research team led by Prof. Chen Wei from the University of Science and Technology of China (USTC) of the Chinese Academy of Science (CAS) revealed for the first time the important role of halogen-mediated solvation structure in the de-solvation process of multivalent ions. The research result was published in Joule.
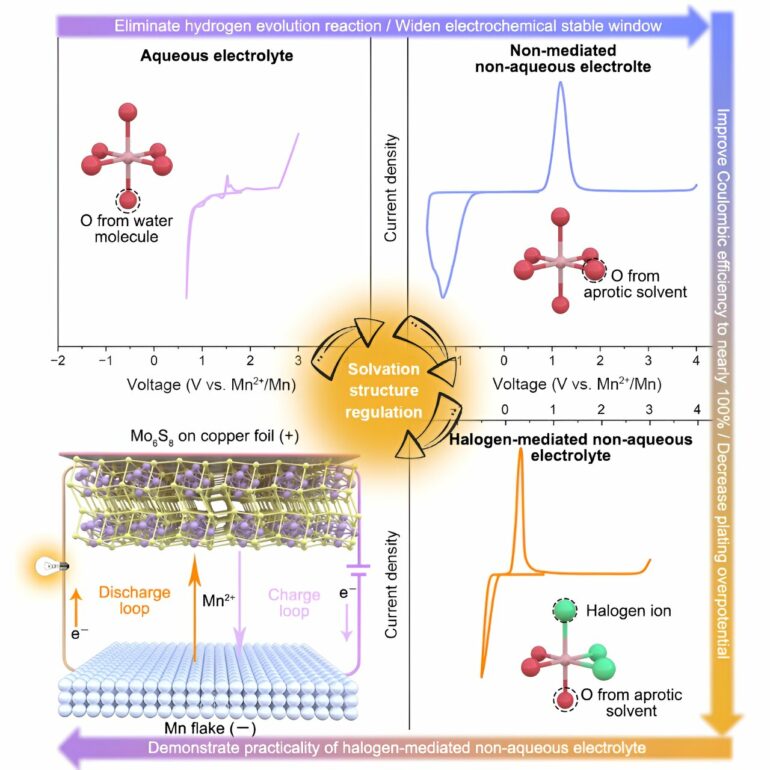
The team managed to use manganese metal batteries (MnMBs) as the research platform to fully demonstrate the important role of halogen-mediated (with Cl as the main research object) mechanism in lowering the overpotential of the multivalent metal ions deposition and enhancing the Coulombic and the dissolution/deposition efficiencies.
With theoretical calculations and experiments, the researchers fully verified that Cl was fully involved in the solvation of Mn2+ in the designed electrolyte, transforming [Mn(Osol)6]2+ solvated structure into [Mn(Osol)3Cl3]2+.
Compared with other atoms, Cl atoms have a larger radius and smaller charge density; thus, the solvated Mn-Cl bond is weaker than the Mn-O bond, greatly reducing the de-solvation energies during deposition, lowering the deposition overpotential of the manganese metal anode and significantly enhancing the Coulombic and Faraday efficiencies.
Researchers assembled symmetric and asymmetric cells to demonstrate the halogen-mediated electrolyte’s reliability. The experimental data showed that the electrolyte can support stable cycling of symmetric cells for more than 700 h at a current density of 0.1mA cm-2, which is far beyond the performance of the reported manganese-metal battery electrolytes.
The symmetric cells showed steady polarization values at different current densities, which fully demonstrated the excellent multiplicity performance of the electrolyte. In addition, the electrolyte provided a Coulombic efficiency close to 100% and deposition/dissolution overpotentials of <200 mV even on different metallic or nonmetallic collectors.
An asymmetric cell assembled from this electrolyte can be stably cycled for more than 1000h when applying Ketjenblack (KB) as the current collector.
Remarkably, the manganese deposition/dissolution efficiency remained as high as 96.8%, even at a higher surface capacity (5 mAh cm-2).
To further validate the value of the designed halogen-mediated electrolyte, the researchers also developed a halogen-meditated non-aqueous manganese metal full cell. The cell was able to cycle stably for nearly 600 cycles in the designed halogen-mediated electrolyte and exhibited satisfying multiplicity performance and two well-defined plateaus.
The study offers a new path to develop rechargeable, non-aqueous MnMBs enabled by electrolyte engineering, which is expected to benefit other multivalent metal batteries and electroplating industries.
More information:
Dongyang Shen et al, A rechargeable, non-aqueous manganese metal battery enabled by electrolyte regulation, Joule (2024). DOI: 10.1016/j.joule.2024.01.012
Provided by
University of Science and Technology of China
Citation:
A rechargeable, non-aqueous manganese metal battery (2024, March 15)