Modern life relies on electricity and electrical devices, from cars and buses to phones and laptops, to the electrical systems in homes. Behind many of these devices is a type of energy storage device, the supercapacitor. My team of engineers is working on making these supercapacitors even better at storing energy by studying how they store energy at the nanoscale.
Supercapacitors, like batteries, are energy storage devices. They charge faster than batteries, often in a few seconds to a minute, but generally store less energy. They’re used in devices that require storing or supplying a burst of energy over a short span of time. In your car and in elevators, they can help recover energy during braking to slow down. They help meet fluctuating energy demand in laptops and cameras, and they stabilize the energy loads in electrical grids.

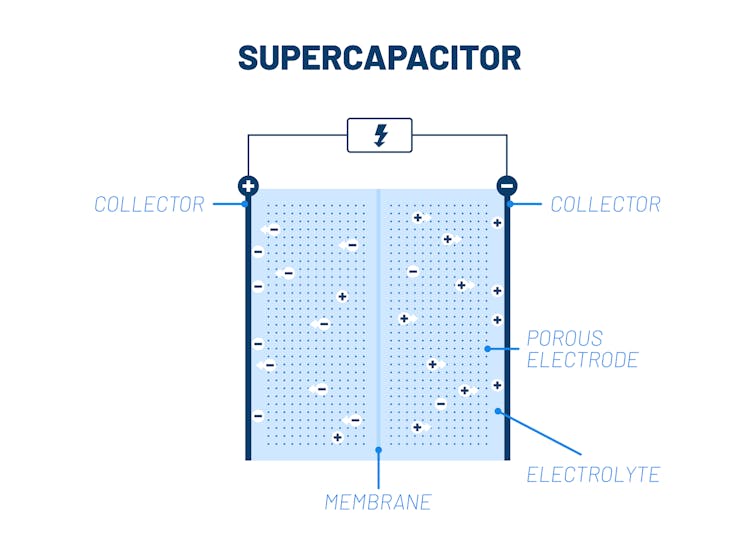
Supercapacitors store energy for use in electronics.
coddy/iStock via Getty Images Plus
Batteries operate via reactions in which chemical species give or take electrons. Supercapacitors, in contrast, do not rely on reactions and are kind of like a charge sponge. When you dip a sponge in water, it soaks up the water because the sponge is porous – it contains empty pores where water can be absorbed. The best supercapacitors soak up the most charge per unit of volume, meaning they have a high capacity for energy storage without taking up too much space.
In research published in the journal Proceedings of the National Academy of Sciences in May 2024, my student Filipe Henrique, collaborator Pawel Zuk and I describe how ions move in a network of nanopores, or tiny pores that are only nanometers wide. This research could one day improve the energy storage capabilities of supercapacitors.
All about the pores
Scientists can increase a material’s capacitance, or ability to store charge, by making its surface porous at the nanoscale. A nanoporous material can have a surface area as high as 20,000 square meters (215,278 square feet) – the equivalent of about four football fields – in just 10 grams (one-third of an ounce) of weight.
Over the past 20 years, researchers have studied how to control this porous structure and the flow of ions, which are tiny charged particles, through the material. Understanding the flow of ions can help researchers control the rate at which a supercapacitor charges and releases energy.
But researchers still don’t know exactly how ions flow into and out of porous materials.
Each pore in a sheet of porous materials is a small hole filled with both positive and negative ions. The pore’s opening connects to a reservoir of positive and negative ions. These ions come from an electrolyte, a conductive fluid.
GettyImages.
Bogdana Pashkevich/iStock via Getty Images Plus
For instance, if you put salt in water, each salt molecule separates into a positively charged sodium ion and a negatively…