
Curious Kids is a series for children of all ages. If you have a question you’d like an expert to answer, send it to [email protected].
What do molecules look like? – Justice B., age 6, Wimberley, Texas
A molecule is a group of atoms bonded together. Molecules make up nearly everything around you – your skin, your chair, even your food.
They vary in size, but are extremely small. You can’t see an individual molecule with your eyes or even a microscope. They are 100,000 times smaller than the width of a hair.
The smallest molecule is made of two atoms stuck together, while a large molecule can be a combination of 100,000 atoms or more. A molecule can be a repeat of the same atom, such as the oxygen molecules we breathe, or can be made up of a variety of atoms, such as a sugar molecule made of carbon, oxygen and hydrogen.
But what do molecules look like? It all begins with their building blocks: atoms.
Opposites attract
The particles of matter that make up an atom are not all the same. They can have a positive charge, a negative charge or no charge. Scientists call them protons, electrons and neutrons.
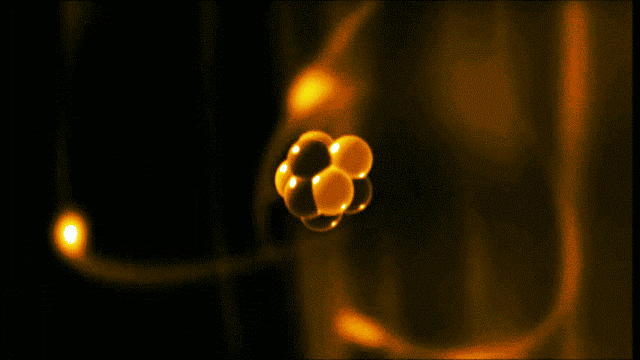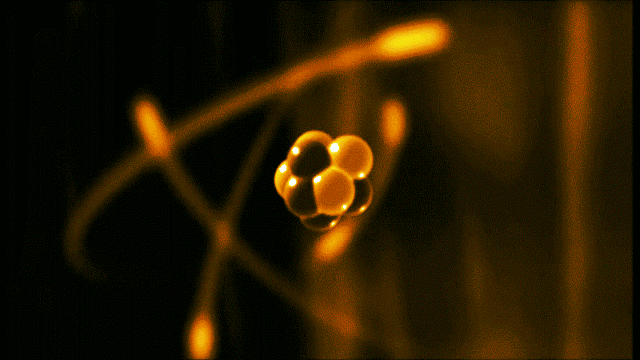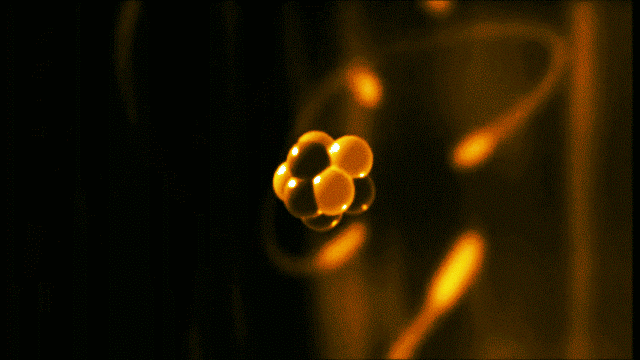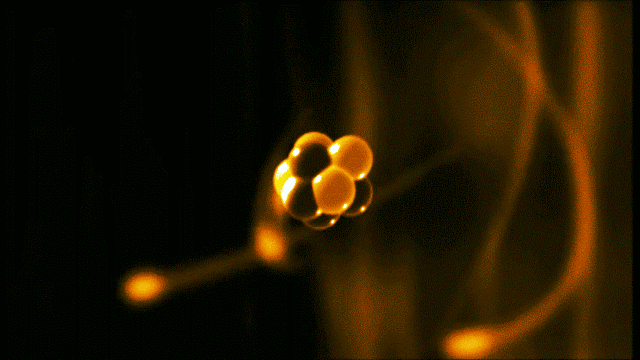
A gold atom has a dense center made of 79 protons and 118 neutrons, with a more-spread-out cloud of 79 electrons around it. Illustration created by Galarza Creador.
Neutrons with no charge and protons with a positive charge form the heavy center of the atom. The negatively charged electrons surround this small center.
As atoms approach each other to potentially join and make molecules, the negative electrons in one atom are attracted to the positive protons in the other, and vice versa. Both atoms adjust themselves accordingly.
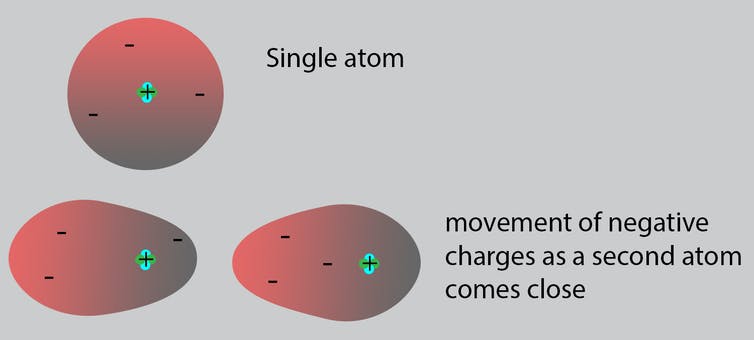
When an atom is alone, the negative electrons surrounding its center are symmetric. As two atoms approach, the negative electrons of one atom move toward the positive center of the other atom.
Christine Helms, CC BY-SA
You can compare it to trying to choose a seat in a classroom. There are some rules. For example, you have to stay in the classroom and you cannot sit on top of someone. Following those rules, you might try to sit next to your friends and far from your enemies. Finding the perfect position so everyone in the class is happy is similar to finding the perfect position for the atoms in a molecule. Sometimes, atoms cannot find a happy arrangement and no molecule is formed.
Seeing the unseeable
If molecules are too small to see with your eyes or even a powerful microscope, how do scientists see them? The answer is they have developed special tools to do it.
One tool uses X-rays, which you might know about since doctors use them to see bones in the body. X-rays are a type of light that human eyes can’t see, like ultraviolet or infrared light.
When scientists shoot X-rays at molecules, some bounce off. Scientists can record these rebounding X-rays and use their patterns to figure out…