Li-ion batteries and other emerging lithium-based battery technologies are currently used to power a wide range of devices, including smartphones, laptops, tablets and cameras. Despite their advantages, batteries containing lithium do not always retain their performance over time.
One of the main reasons for the performance decay observed in some Li-based batteries is that the lithium contained within them sometimes becomes inactive or “dead.” This “dead lithium” can cause capacity decay and thermal runaway, which can ultimately reduce a battery’s lifespan and impair its performance.
Researchers at Zhejiang University of Technology in China and Argonne National Laboratory in the U.S. have recently devised a strategy to restore inactive lithium in Li metal anodes. This strategy, outlined in a paper published in Nature Energy, is based on a chemical reaction known as iodine redox.
The solid-electrolyte interphase (SEI) is a layer that is produced on the anode of lithium-ion batteries during the first few charging cycles. This passivating layer plays a crucial role in ensuring the efficiency, stability and safety of batteries.
In a typical Li-ion battery cell with a conventional graphite anode, the solid-electrolyte interphase (SEI) is typically comprised of LiF, combined with Li2CO3, alkyl carbonate and other substances. Recent studies have shown that in batteries with a Li metal anode, the SEI is primarily made of Li2O, rather than LiF. In these batteries, the variation in the Li plating’s volume can compromise the mechanical integrity and passivating role of the Li2O-based SEI. This can in turn lead to the formation of “dead lithium.”
Past studies have proposed a number of strategies to improve the performance of batteries with Li metal anodes. For instance, some researchers tried to use artificial SEI structures or SEI-regulated electrolyte additives, such as cesium hexafluorophosphate or fluoroethylene carbonate. However, they found that the volume variation of the Li plating causes the SEI to break, exposing the lithium to the electrolyte and leading to the formation of a new SEI. This continuous SEI damage and repair can impact a battery’s performance over time.
In their paper, Chengbin Jin his colleagues at Zhejiang University and Argonne National Laboratory tried to quantify the amount of Li2O in the SEI layer formed on Li metal anodes. In addition, they investigated the role of inactive SEI’s in the production of electrically isolated dead Li metal.
“A fundamental solution for recovering dead lithium is urgently needed to stabilize lithium metal batteries,” the researchers wrote in their paper. “We quantify the SEI components and determine their relation with the formation of electrically isolated dead lithium metal.”
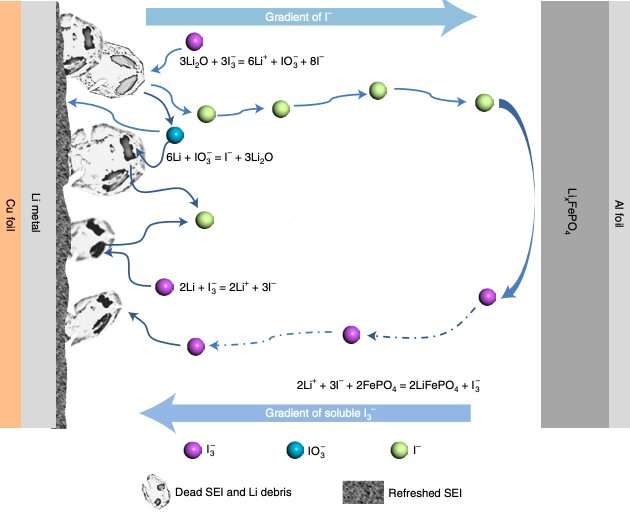
The findings gathered by Jin and his colleagues suggest that a loss of Li in the SEI and dead Li debris are the main causes for the performance decay often observed in Li metal batteries. This observation inspired them to devise a method to restore dead lithium, utilizing a iodine redox chemical reaction.
“We present a lithium restoration method based on a series of iodine redox reactions mainly involving I3 −/I−,” the researchers explained in their paper. “Using a biochar capsule host for iodine, we show that the I3 −/I− redox takes place spontaneously, effectively rejuvenating dead lithium to compensate the lithium loss.”
Using the design strategy that they developed, Jin and his colleagues were able to create a full battery cell with very little lithium in the anode. This cell had a remarkable lifespan of 1,000 cycles and achieved a high Coloumbic efficiency of 99.9%.
To evaluate the effectiveness of their Li restoration strategy further, the researchers combined the anode with commercial cathodes (i.e., LiFePO4 and LiNi0.8Co0.1Mn0.1O2) and created a pouch cell. This cell achieved very promising results, both in terms of lifecycle and efficiency.
In the future, the strategy devised by this team of researchers could inform the development of new, better performing batteries with Li metal anodes. In addition, it could be used to extend the cycling lifetimes of existing Li-ion cells and other Li-based battery technologies.
First closeups of how a lithium-metal electrode ages
More information:
Rejuvenating dead lithium supply in lithium metal anodes by iodine redox. Nature Energy(2021). DOI: 10.1038/s41560-021-00789-7
2021 Science X Network
Citation:
A strategy to rejuvenate dead lithium inside batteries (2021, April 22)
retrieved 22 April 2021
from https://techxplore.com/news/2021-04-strategy-rejuvenate-dead-lithium-batteries.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.