The electrical potential across the bacterial cell envelope indicates when bacteria no longer operate as individual cells but as a collective. Researchers at the University of Cologne’s Institute for Biological Physics have discovered this connection between the electrical properties and the lifestyle of bacteria.
Although bacteria are single cellular organisms, they form spatially structured communities, so-called biofilms. Within biofilms, bacteria behave as a collective and can protect themselves better against external stresses like antibiotics. Until now, it was largely unknown how the transition from a single bacterium to such a complex community works.
The researchers examined how the electrical properties of bacteria change during biofilm formation and discovered characteristic patterns of the electrical potential that evolve in space and time. These patterns correlated with the development of new habitats with varying degrees of tolerance to antibiotics. The researchers describe their findings in the article “Collective polarization dynamics in bacterial colonies signify the occurrence of distinct subpopulations” in the journal PLOS Biology.
Single bacteria build up an electric potential across their envelope (the membrane), and thus are electrically polarized. For the cell, this polarization is an important energy source for respiration, the uptake of nutrients and the export of toxins. Recent methodological advances have enabled researchers to examine the dynamics of the membrane potential at the scale of single bacterial cells. These studies revealed that the membrane potential of single cells fluctuates independent of their neighboring cells.
How does the potential change during biofilm development, and which environmental factors influence the potential? How does the potential relate to growth behavior of cells and their tolerance to antibiotics? These questions have now been posed by a team of researchers at the Institute for Biological Physics led by Professor Berenike Maier.
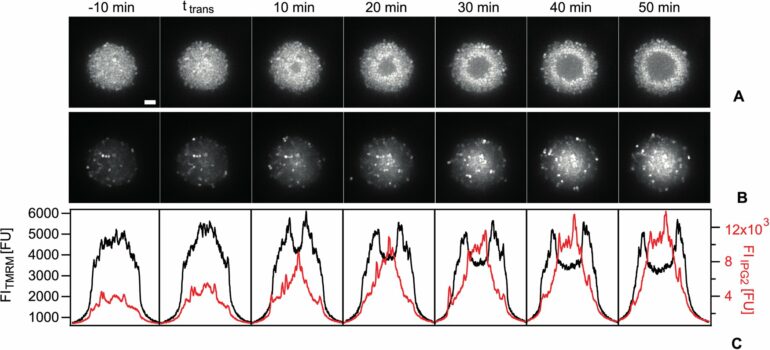
They examined early stages of biofilm formation of Neisseria gonorrhoeae (aka gonococcus), the causative agent of gonorrhea, one of the most common sexually transmitted diseases, which can cause ectopic pregnancies and infertility. Within a few minutes, gonococci self-assemble into spherical colonies that comprise thousands of bacteria.
“Using advanced light-microscopy and image analysis, we can measure the dynamics of the membrane potential of single cells in these colonies,” the first author Dr. Marc Hennes explains. “The potential is uncorrelated within fresh colonies in bacteria. When the colony reaches a critical size, we observe something completely unexpected: All the cells in the center suddenly increase their potential; they hyperpolarize.” Eventually, a shell of hyperpolarized cells occurs at the colony center and travels through the colony. Behind this shell, the potential at the center is lower.
The researchers have interpreted this phenomenon of spatiotemporal correlated polarization patterns as the transition towards collective behavior, indicative of biofilm formation. A combination of computer simulations and wet lab experiments showed strong evidence that this polarization pattern is linked to a change in the availability of oxygen. This pattern exists because cells at the center deplete the oxygen faster than diffusion resupplies it.
An important question, therefore, was whether the pattern of membrane polarization correlated with the well-known functional heterogeneity of biofilms. Indeed, bacteria decreased their growth rate after they had gone through the process of hyperpolarization, whereas the growth rate of bacteria residing on the surface of the colony remained high.
In addition, bacteria in the center of the colony showed more tolerance towards antibiotics. Increased tolerance to antibiotics is an acute medical problem when treating biofilms. The molecular mechanisms of tolerance are the subject of a new project. The future goal is to better understand the molecular mechanisms that underlie the formation of polarization patterns and their relation to antibiotic tolerance.
More information:
Marc Hennes et al, Collective polarization dynamics in bacterial colonies signify the occurrence of distinct subpopulations, PLOS Biology (2023). DOI: 10.1371/journal.pbio.3001960
Provided by
University of Cologne
Citation:
Electrical properties of bacteria: How membrane potential influences antibiotic tolerance (2023, January 19)