The more we learn about the biological world, the more complex it becomes.
Nowhere is this more apparent than in recent discoveries about the ways in which microorganisms influence their hosts.
The composition of the microbiome—that is, the community of fungi, bacteria and viruses that usually exist in the gut—can affect the health of their host organisms. This includes their ability to fight disease, the level of nutrition they can extract from food and more.
The influence of the microbiome is particularly dramatic in the case of insects where microorganisms can exist not only in the gut of the host insect, but also inside the host’s cells. These intracellular microorganisms include bacteria that are passed on from mothers to their offspring.
These types of bacteria are known as endosymbionts and can have a whole range of effects on their hosts.
These may include effects that help the host, like the provision of nutrition to cells and protecting the host cells against viruses. But they also have more dubious results—including killing the host’s embryos and changing the sex ratio of offspring the host produces.
When the endosymbionts are detrimental to the host, the host genome might evolve to block these effects. This can lead to an evolutionary “arms race” between the host and its bacterial endosymbiont.
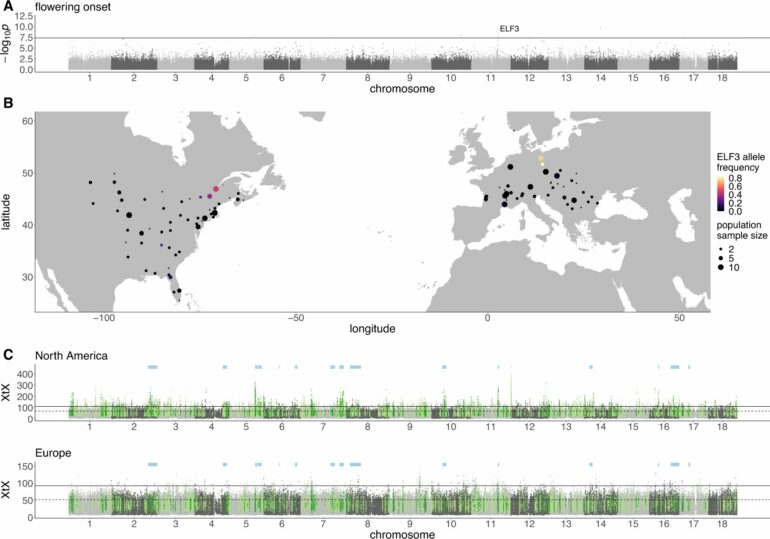
Our research team has now uncovered a remarkably complex example of processes like this in an endemic Australian fly known as Drosophila pseudotakahashii, which is common along the east coast of Australia.
Our work, published in the journal PLOS Biology, initially found that all members of this fly carry an endosymbiont bacteria known as Wolbachia, which is passed from the mother to their offspring.
When we use antibiotics to cure flies of this Wolbachia strain, the flies survive. However, in any mating that takes place between males that were infected by Wolbachia and females that no longer carry the infection—the offspring of these females die.
This results in an enormous advantage to the females that carry the infection because they are not sterilized by infected males.
This whole situation is made more complicated by the fact that a second Wolbachia strain exists that can infect flies alongside the first strain.
This arms race produced very rapid changes in a specific region of the fly’s genome. © Tank Monsternova
When the second strain is present, female flies only produce female offspring—making it effectively a “male-killer.”
In some cases, this might benefit the flies by reducing competition for resources and helping them to avoid inbreeding. But male-killers can mean a big cost for the fly, because flies cannot produce offspring without mating.
In the absence of males, the fly population would become extinct.
So, this sets up another arms race where the host must evolve a way of suppressing the male-killer bacteria.
In Drosophila pseudotakahashii, we saw this arms race in action, producing very rapid changes in a specific region of the fly’s genome—which is the complete set of DNA (genetic material) in an organism.
And the genome began to win.
Over a few weeks, the fly populations with the male-killer bacteria were able to start producing males again, despite the presence of the second Wolbachia strain. And we were able to identify some genes that are likely to be involved in this “male-killer suppression.”
Our local fly species can provide us with a lesson in the complex nature of interactions between hosts and microorganisms that shape many aspects of an organism’s life. Moreover, it illustrates the ongoing evolutionary changes that can happen very quickly to alter these internal interactions.
But these specific lessons have broader implications as we look toward manipulating the microbiome of organisms to encourage beneficial effects.
For instance, Wolbachia strains living inside mosquitoes are being deliberately spread around the world because the bacteria can actually block the transmission of some mosquito-borne diseases like Dengue Fever.
But what if the host’s genome evolves to stop these effects? Or what if bacteria that kill males could be released to suppress pest populations?
We are at the start of a journey when it comes to microorganisms and the more we learn, the more we need to expect the unexpected.
More information:
Kelly M. Richardson et al, A male-killing Wolbachia endosymbiont is concealed by another endosymbiont and a nuclear suppressor, PLOS Biology (2023). DOI: 10.1371/journal.pbio.3001879
Provided by
University of Melbourne
Citation:
Genome of Australian fly defeats killer bacteria by evolving to co-exist with it (2023, March 28)