Researchers at Vanderbilt University and the University of Arizona have revealed more of the inner-workings of a two-stage “molecular motor” in the cell membrane that enables bacteria to become resistant to drugs.
Their findings, which were reported recently in the journal Nature Chemical Biology, will aid the search for inhibitors that can “turn off” the protein, called an ABC transporter. They also inform efforts to block the human version of the transporter that enables tumor cells to become resistant to chemotherapy.
“We’re piecing together this story from different transporters and asking the question, how did nature design this particular molecular motor?” said Vanderbilt’s Hassane Mchaourab, Ph.D., co-corresponding author of the paper with Thomas Tomasiak, Ph.D., of the University of Arizona in Tucson.
Understanding how transporters work is essential to developing drugs to block them, he said.
A national leader in the study of protein dynamics, Mchaourab is the Louise B. McGavock Professor in the Department of Molecular Physiology & Biophysics at Vanderbilt. Tomasiak, who earned his Ph.D. in Pharmacology at Vanderbilt in 2011, is assistant professor of Chemistry and Biochemistry at the University of Arizona.
According to the U.S. Centers for Disease Control and Prevention, antibiotic-resistant bacteria and fungi infect at least 2.8 million people in the United States each year and are responsible for more than 35,000 deaths.
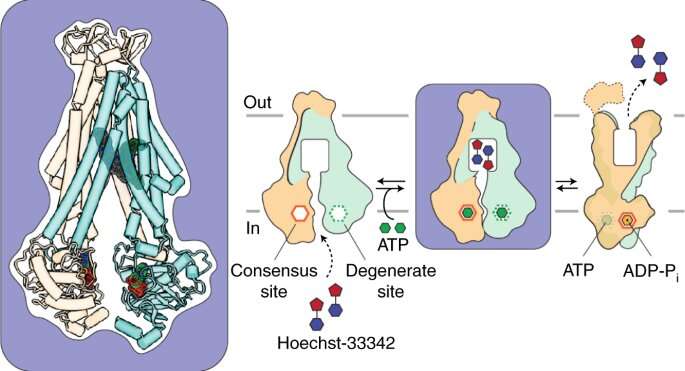
A primary vehicle for resistance is the multi-drug ABC (ATP-binding cassette) exporter. ABC exporters use ATP hydrolysis—the release of chemical energy stored in ATP molecules—to traffic a wide variety of molecules across cell membranes.
ATP energy provides the power for ABC exporters to bind toxic chemicals, then turn around and expel them from the cell. In the case of antibiotic-resistant bacteria, however, this survival tactic can prove deadly to the human host they have invaded.
In 2014, Mchaourab’s team, in collaboration with researchers at the University of Illinois at Urbana-Champaign, used a technique called electron paramagnetic resonance (EPR) spectroscopy to identify previously unreported changes in the shape, or conformation, of the ABC exporter from a bacterium called Bacillus subtilis as it interacts with ATP.
They proposed that ATP power, in a series of complex steps, drives the transition between inward-facing and outward-facing conformations of the exporter. After binding the antibiotic, for example, the exporter “turns around” so it can expel its cargo from the cell.
This motion is driven by the transduction (conversion) of chemical energy into mechanical energy resulting from asymmetrical and sequential binding of two ATP molecules to different parts of the protein complex (the ATP binding cassettes). Asymmetrical binding thus drives conformational change.
To prove their theory, the researchers had to capture an image of the conformational change. So they turned to another resource, cryogenic electron microscopy, which enables measurement of atomic distances at cryogenic temperatures, below minus 320 degrees Fahrenheit.
The cryo-EM studies were conducted at the Pacific Northwest Center for Cryo-EM in Portland, Ore. In combination with an EPR spectroscopy method called DEER and molecular dynamics simulation, the studies revealed for the first time an ATP-loaded, inward-facing structure with two drug molecules bound asymmetrically.
“In 2014, we had indirect evidence,” Mchaourab said. “Now, with cryo-EM, we can say, “Here in atomic detail is what is going on.'”
The intermediate conformation suggests that drugs could be designed to prevent the bacterial exporter from turning around and expelling the antibiotic by “trapping” it in its inward-facing state, he added.
More information:
Tarjani M. Thaker et al, Asymmetric drug binding in an ATP-loaded inward-facing state of an ABC transporter, Nature Chemical Biology (2021). DOI: 10.1038/s41589-021-00936-x
Provided by
Vanderbilt University
Citation:
Study explores how bacteria become drug resistant (2022, January 7)