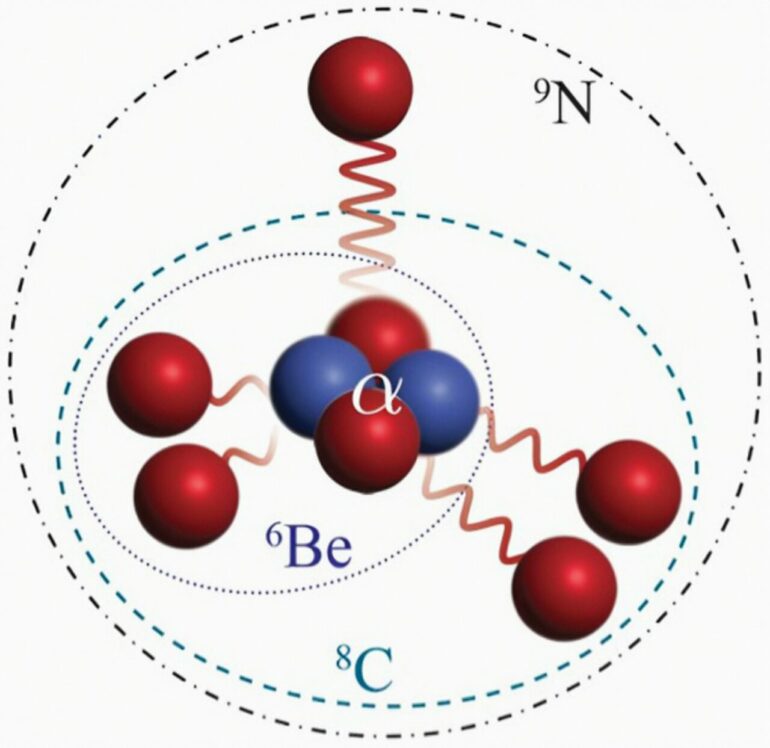
With only two neutrons to its seven protons, Nitrogen-9 represents the first known case of a nucleus that decays by emitting five protons from its ground state. Robert Charity, a research professor of chemistry in Arts & Sciences at Washington University in St. Louis, described the new light isotope of nitrogen in a new paper published in Physical Review Letters.
The highly ephemeral Nitrogen-9 nuclide is located on the proton-rich edge of the Chart of Nuclides and is close to the limit of what can be considered a nuclear state. Charity and his collaborators obtained the experimental evidence for Nitrogen-9 from data collected at the National Superconducting Cyclotron Laboratory.
Nitrogen-9 can be viewed as five unbound protons which surround an alpha-particle (a tightly-bound grouping of two protons and two neutrons). This ensemble is briefly held together by their mutual interactions. The unbound protons are liberated in steps, with an initial single proton escaping followed by the simultaneous emission of a first, and then slightly later, a second pair of protons.
“The decay of Nitrogen-9 is like opening a set of nesting dolls; each decay reveals another nuclide, which also decays by the emission of a single or a pair of protons,” Charity said. “The existence of such an exotic system is a good test of the quantum mechanics of open or unbound many-body systems.” This WashU-led collaborative research effort included theorists in open quantum systems from Michigan State University and Fudan University in China.
Concurrent with their description of Nitrogen-9, Charity and his collaborator Lee Sobotka, a professor of chemistry and of physics in Arts & Sciences, published a paper in Physical Review C that describes how to analyze the types of experiments that yield results on truly exotic nuclei.
“The elements we have around us are made via a set of mechanisms that work through intermediates that we do not have around us,” Sobotka said. “These intermediates are unstable and often have highly unusual neutron-to-proton ratios. Our work involves both reconstructing the structure of, and reactions producing, such nuclei.”
Learning about such unstable nuclear configurations and the reactions that produce them allows scientists to understand why we have the isotopes that exist on Earth, and why some are plentiful and others rare.
More information:
R. J. Charity et al, Strong Evidence for N9 and the Limits of Existence of Atomic Nuclei, Physical Review Letters (2023). DOI: 10.1103/PhysRevLett.131.172501
R. J. Charity et al, Invariant-mass spectroscopy in projectile fragmentation reactions, Physical Review C (2023). DOI: 10.1103/PhysRevC.108.044318
Provided by
Washington University in St. Louis
Citation:
Strong evidence for new light isotope of nitrogen (2023, October 30)