Bisphenol A, or BPA, is a chemical widely used to make hard, clear plastics. It is an endocrine disruptor that has been linked to many negative health effects, including cardiovascular diseases and diabetes. In 2013, the U.S. government banned its use in baby products that come into contact with food, like bottles or the packaging of infant formula.
At the time, the U.S. Food and Drug Administration concluded that some exposure was safe for adults. But other health agencies, including the European Food Safety Authority, have concluded that the levels of BPA the FDA considers safe may have adverse health effects for adults as well.
In early June 2022, the FDA signaled that it is reconsidering what amount of exposure to BPA is safe for adults, announcing that it would reconsider its guidance on the use of BPA in plastics that come into contact with food.
As a synthetic polymer chemist, I think a lot about how to design new polymers, with particular focus on how to do so sustainably. It’s natural to wonder why companies don’t simply replace BPA with another chemical if health is such a concern. The secret to what makes BPA such an irreplaceable ingredient in plastics is the same thing that leads to its health risks – the molecule’s chemical structure.
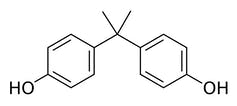
Bisphenol A is made of two carbon rings with small alcohol groups attached and is used to produce strong, clear plastics.
Darkness3560/Wikimedia Commons
What is BPA?
BPA is a small molecule made of two carbon rings with a bonded oxygen and hydrogen attached to either end. BPA can react with other carbon-based molecules to form long chains, with the BPA molecules stitched together by small chemical links.
Nearly all of the BPA produced in the world is used to manufacture plastics, mostly a specific type called polycarbonate. BPA-derived polycarbonates are transparent, incredibly strong, light and don’t begin to melt or lose structural integrity until they reach very high temperatures. These properties make polycarbonates excellently suited for use in everything from the lenses of eyeglasses to water bottles.
It’s all about the structure
In chemistry, structure means everything. The reasons different materials have different properties is due to their chemical structure.
BPA polymers are rigid because the carbon rings in BPA molecules are themselves rigid. Compare this to polyethylene, the thin, flexible material used to make plastic bags. The long chains of repeating molecules that make up polyethylene are very flexible. So the plastics they produce are highly pliable, too.

BPA plastics are strong, transparent, light and have a high melting point, which makes them the perfect material for lenses for your eyeware.
Nipitphon Na Chiangmai / EyeEm via Getty Images
How do BPAs leach out of plastic?
When BPA plastics are made, nearly all the individual molecules of BPA are chemically bound to the…
