The 2022 Nobel Prize in chemistry was awarded to scientists Carolyn R. Bertozzi, Morten Meldal and K. Barry Sharpless for their development of click chemistry and bioorthogonal chemistry.
These techniques have been used in a number of sectors, including delivering treatments that can kill cancer cells without perturbing healthy cells as well as sustainably and quickly producing large amounts of polymers to build materials. One click chemistry-based drug is currently undergoing phase 2 clinical trials. Bertozzi is a scientific adviser of the company developing the drug.
We asked chemistry Ph.D. candidate Heyang (Peter) Zhang of the Lin Lab at the University at Buffalo to talk about how these techniques figure in his own research and how they have transformed his field and other industries.
1. How does click and bioorthogonal chemistry work?
Click chemistry, as the name suggests, is a way of building molecules like snapping Lego blocks together. It takes two molecules to click, so researchers refer to each one as click partners.
K. Barry Sharpless and Morten Meldal independently discovered that azide, a high-energy molecule with three nitrogens bonded together, and alkyne, a relatively inert and naturally rare molecule with two carbons triple-bonded together, are great click partners in the presence of a copper catalyst. They found that the copper catalyst can bring the two pieces together in an optimal arrangement that snaps them together. Prior to this technique, researchers did not have a way to quickly and precisely make new molecules under accessible conditions, like using water as a solvent at room temperature.
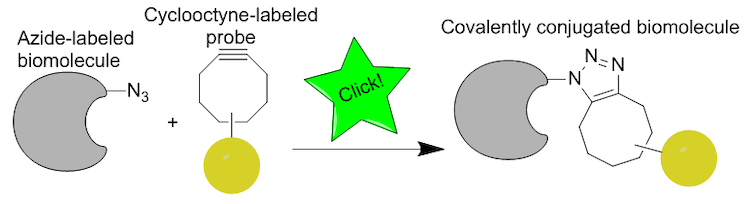
By combining an azide with a cyclooctyne, bioorthogonal chemistry allows researchers to join molecules quickly together without disturbing the rest of the cell.
Cliu89/Wikimedia Commons
Chemical biologists quickly realized that click reactions can be a fantastic way to probe living systems like cells because they produce little to no toxic byproducts and can happen quickly. However, the copper catalyst is itself toxic to living systems.
Carolyn Bertozzi devised a workaround for this issue by removing the copper catalyst from the reaction. She did this by placing the alkyne into a ring structure, which drives the reaction forward using the ring strain produced from molecules forced into a cyclical shape. These bioorthogonal reactions, or reactions that happen “parallel” to the chemical environment of the cell, can occur in cells without perturbing their normal chemistry.
2. How do you use this chemistry in your work?
In an interview, Carolyn Bertozzi stated that the next steps for bioorthogonal chemistry are to find new reactions and applications for it. Our lab’s research focuses exactly on that.
My colleagues and I apply this technique to track molecules we are interested in as they naturally behave in a cell. In a living cell, we were able to add a probe to a receptor…